- Title
-
Wnt/beta-catenin and Fgf signaling control collective cell migration by restricting chemokine receptor expression
- Authors
- Aman, A., and Piotrowski, T.
- Source
- Full text @ Dev. Cell
|
Constitutive Activation of Wnt/β-Catenin Signaling Disrupts Primordium Migration, but Not Cell Type Specification Anterior is oriented toward the left in all figures. (A and B) In situ hybridization with the lateral line marker eya1 of 36 hpf WT and apcmcr mutant embryos. (A) In WT embryos, the primordium (asterisk) has deposited neuromasts (arrows) and almost reached the tail tip. (B) In mutant embryos, the primordium (asterisk) stopped migrating before reaching the tail. (C?E) Still images of a 90 min time-lapse recording of a normally migrating lateral line primordium in a Tg(Cldnb:lynGFP) embryo. (C) 34 hpf, time point 0 min. (D) After 45 min. (E) After 90 min. The trailing zone of the primordium (arrow) has almost left the field of view. (F?H) Still images of a 90 min time-lapse recording of a Tg(Cldnb:lynGFP);apcmcr mutant primordium. (F) 34 hpf, time point 0 min. (G) After 45 min. (H) After 90 min. The primordium is not moving tailward. (I and J) Higher-magnification images of tip cells (arrows) in migrating (I) Tg(Cldnb:lynGFP) and (J) Tg(Cldnb:lynGFP);apcmcr mutant primordia. (K?N) (K and L) Support and (M and N) hair cells are specified in apcmcr mutant embryos. klf4 in situ labels supporting cells in 36 hpf (K) WT and (L) apcmcr mutants. Hair cells in (M) Tg(Brn3c:GAP43-GFP)s356t and (N) Tg(Brn3c:GAP43-GFP)s356t;apcmcr 72 hpf mutant embryos. (O) Simplified protein structure of WT Apc, Apcmcr, and APC-GFP. (P) An apcmcr embryo that was rescued by APC-GFP injection as revealed by eya1 in situ. Arrows indicate neuromasts; an asterisk labels the primordium. (Q) Quantification of the rescue effect. Rescue was found to be significant by a test of difference in population proportions (p = 2.3 x 10-7). (R?W) (R and U) Wnt target genes lef1 and axin2 are expressed in the tip of 36 hpf WT primordia. (S and V) In apcmcr mutant embryos, lef1 and axin2 are expressed in the entire primordium and deposited cells. (T and W) Tg(hs:Dkk1) embryos, heat shocked at 20 hpf, express no lef1 or axin2 by 4 hr post-heat shock. Scale bars in (A)?(L) and (R)?(W) are equal to 40 μM. Scale bars in (M) and (N) are equal to 20 μM. EXPRESSION / LABELING:
PHENOTYPE:
|
|
apcmcr Mutant Cells Display Cell-Autonomous Defects and Exert a Non-Cell-Autonomous Effect on Neighboring WT Cells (A) Mosaic embryo generated by transplanting red fluorescently labeled apcmcr cells into Tg(Cldnb:lynGFP) embryos. (B and C) Higher-magnification views of the boxed areas in (A). (B) Red mutant cells are deposited and form proneuromasts in ectopic positions (arrows). (C) apcmcr cells induce morphogenesis defects in neighboring green WT cells. (D) Overview of an embryo generated by transplanting fluorescently labeled WT cells into apcmcr mutant embryos. (E and F) Higher-magnification images of two additional WT to apcmcr transplantations. |
|
Wnt/β-Catenin Signaling Regulates Fgf Signaling in the Migrating Primordium (A?H) fgf3 and fgf10 are restricted to the leading zone of (A and E) WT primordia and are upregulated in (B and F) apcmcr mutant primordia. (C and G) Their expression is lost in the absence of Wnt/β-catenin signaling. (D and H) fgf3 and fgf10 are upregulated in the absence of Fgf signaling. (I) pea3 expression in WT primordia shows that Fgf signaling is only active in the trailing cells. (J and K) pea3 is expanded in 38 hpf (J) apcmcr mutants and lost in (K) Tg(hs:Dkk1) embryos. (L) pea3 expression is abolished by SU5402 treatment. (M) The Fgf pathway inhibitor sef is expressed in the leading zone of WT primordia. (N and O) sef expression is expanded in (N) apcmcr mutants and abolished in (O) Tg(hs:Dkk1) embryos. (P) sef expression does not require Fgf signaling, as it is present in SU5402-treated primordia. (Q?V) (S and T) Injection of sef morpholino (MO) disrupts primordium migration, (U and V) although the primordia orient correctly toward the posterior. (Q and R) pea3 expression expands into the leading region of sef morphant primordia. WT, apcmcr, and SU5402-treated embryos were fixed between 32 and 36 hpf, Tg(hs:Dkk1) embryos were heat shocked at 26 hpf and fixed at 32 hpf, and sef morphant embryos were fixed at 26 hpf. Brackets in (B), (F), (G), and (N) indicate the primordium. Scale bars in (A)?(P) and (Q), (R), (U), and (V) are equal to 40 μM. Scale bars for (S) and (T) equal 100 μM. |
|
Wnt/β-Catenin-Mediated Fgf Signaling Is Necessary for Neurogenesis and Rosette Formation (A and B) (A) The trailing zone of WT primordia contains proneuromasts distinguishable by their rossette morphology, focal accumulation of Claudin-GFP (arrows), and expression of the proneural gene (B) atoh1 at 32 hpf. (C and D) (C) Loss of Fgf signaling by treatment with SU5402 between 20 and 38 hpf causes loss of rosette formation and (D) atoh1 expression. (E and F) (E) Similarly, abrogating expression of Fgf ligands by inhibiting Wnt/β-catenin via heat shock induction of Dkk1 at 20 hpf leads to a loss of rosettes and (F) atoh1 expression at 28 hpf. (G and H) Still images of Movie S4. Ectopic rosette formation does not contribute to the apcmcr migration phenotype, as stalled mutant primordia still have normally unpatterned leading zones (bracket in [G]). Ectopic rosette formation occurs in the leading zone at 5 hr after stalling (bracket in [H]). (I?N) dkk1 disrupts neuromast (NM) deposition without affecting migration. (I) At 24 hpf, the WT primordium is migrating, but no NM deposition has occurred. (J) dkk1 induction at 24 hpf leads to the formation of a small L2 and complete loss of more posterior NMs without affecting migration, as evidenced by the presence of the lateral line nerve (white arrow). (K) By 28 hpf, the WT primordium has deposited two NMs, L1 and L2. (L) dkk1 induction at 28 hpf ablates the NMs posterior to L3. (M) Quantification of NM numbers for dkk1 induction at 24 hpf and 28 hpf. On average, the primordium is able to deposit one additional NM after dkk1 induction (orange bars). Data are shown as means ± SD (*p << 0.001, **p << 0.001, ?p << 0.001 Student's t test). (N) At 48 hpf, the WT primordium has deposited all posterior NMs. |
|
Fgf Signaling Inhibits Wnt/β-Catenin Signaling via Induction of dkk1 (A and B) SU5402 treatment leads to ectopic induction of the Wnt/β-catenin signaling targets lef1 and axin2 in 36 hpf embryos. (C) In WT embryos, dkk1 is expressed adjacent to the unpatterned tip of the primordium. (D?F) (D) dkk1 is a Fgf target, as it is highly upregulated in apcmcr mutant primordia and is absent in (E) Fgf signaling-depleted WT, as well as in (F) Fgf signaling-depleted apcmcr mutant primordia. (G and H) Morpholino knockdown of dkk1 causes expansion of lef1 and axin2, similar to loss of Fgf signaling. The scale bar is equal to 40 μM. |
|
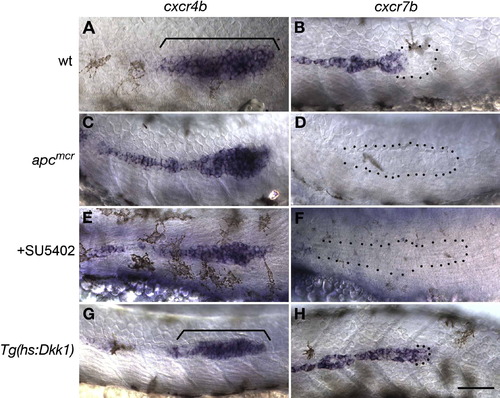
Localized Wnt/β-Catenin Signaling Is Necessary for Asymmetric Expression of Chemokine Receptors (A and B) In 36 hpf WT embryos, (A) cxcr4b is restricted to the leading zone, and (B) cxcr7b is restricted to the trailing zone of the migrating primordium. (C?F) Expanded Wnt/β-catenin signaling in apcmcr mutants and SU5402-treated embryos leads to (C and E) expansion of cxcr4b and (D and F) loss of cxcr7b. (G) cxcr4b expression is not affected by a loss of Wnt/β-catenin signaling. (H) Loss of Wnt/β-catenin signaling leads to expansion of cxcr7b into the leading zone of the primordium. The scale bar is equal to 40 μM. |
|
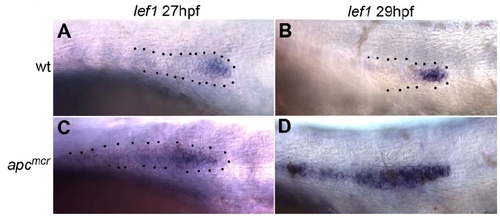
apcmcr Mutant Primordia Stall Concomitantly with Changes in Gene Expression Between 27hpf and 29hpf In wt primordia lef1 is restricted to the leading zone of the primordium at 27hpf (A) and 29hpf (B). In apcmcr mutant primordia lef1 is initially restricted from trailing cells at 27hpf (C) but is fully expanded into the trailing zone by 29hpf (D). |
|
Abrogation of Fgf Signaling Leads to a Primordium Migration Defect Very Similar to the Defect Observed in apcmcr Mutant Embryos (A) In situ hybridization with eya1 at 38hpf. Primordium migration occurs normally and neuromasts are deposited (arrows) in wt embryos that have been incubated in DMSO between 20-38 hpf. (B) In wt embryos that have been incubated in DMSO and SU5402 the primordium stalls and fails to migrate to the tip of the tail. Scale bar represents 40μM. |
|
fgfr1 Is a Feedback Target of Fgf Signaling in the Primordium fgfr1 expression is restricted from the leading zone throughout wt primordium migration (A,B). fgfr1 is progressively upregulated in the leading zone of stalled apcmcr mutant primordia between 32 and 38hpf (C,D). fgfr1 expression is lost in embryos treated with SU5402 from 20-38hpf (E). |
|
sef is a Primordium-Specific Wnt/β-Catenin Target (A) In 32hpf wt embryos sef is absent in trailing cells in the primordium and in deposited cells (arrow). (B and C) (B) 32 hpf apcmcr mutants expresses sef ectopically in all lateral line cells (arrow), although global expression appears overtly normal. In Fgf inhibited 38hpf embryos sef is upregulated in trailing cells and is downregulated in other regions of the embryo, such as the pharyngeal arches (white asterisk) (C). |
|
sef Knockdown Leads to Ectopic Expression of dkk1 in the Leading Zone (A) dkk1 is expressed in a region immediately adjacent to the leading zone in wt primordia. (B) In primordia of 26hpf sef morphant embryos dkk1 is ectopically expressed in tip zone cells consistent with a role for sef in repressing Fgf signaling in these cells. |
|
spry4 is an Fgf Target in the Primordium (A) spry4 is expressed in the trailing zone of 32hpf wt primordia where Fgf signaling is active. (B) Expression is abolished in primordia exposed to the Fgf pathway inhibitor SU5402 from 20-38hpf. (C) Like other Fgf targets, spry4 is expressed throughout the apcmcr mutant primordium at 38hpf. |
|
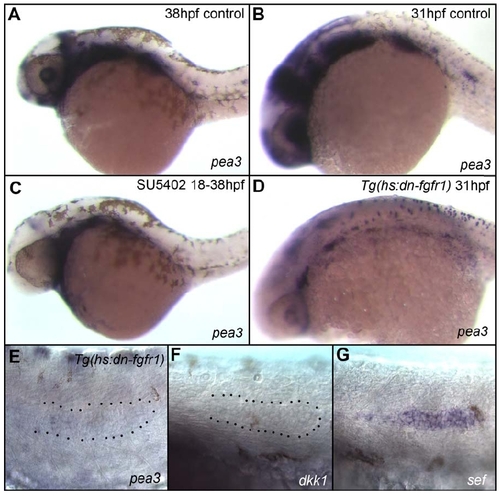
Induction of Dominant Negative Fgfr1 Phenocopies Fgf Inhibition via SU5402 Treatment (A and B) In wt embryos the Fgf target pea3 is expressed in the lateral line as well as many other structures. Expression of pea3 is strongly downregulated or completely abolished in embryos treated with SU5402 between 18-38hpf (C), and in 31hpf Tg(hs:dn-fgfr1) embryos at 4h post heat-shock (D). (E) Higher magnification image of the primordium of embryo shown in (D). Expression of the Fgf target dkk1 (F) is also abolished 4 hours following heatshock induction of dn-Fgfr1. (G) The Fgf pathway repressor sef is ectopically expressed in the trailing edge 4 hours following induction. |
|
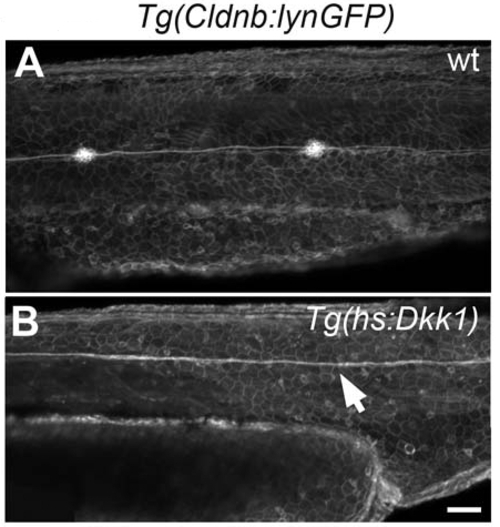
Loss of Neuromasts in Tg(hs:Dkk1) Is Confirmed by Confocal Imaging (A) In 48hpf Tg(claudinb:gfp) embryos the primordia have migrated to the tail tip and deposited neuromasts. (B) Heatshocking Tg(hs:Dkk1) primordia at 26hpf leads to loss of posterior neuromasts at 48 hpf without affecting migration evidenced by the presence of the lateral line nerve (white arrow). |
|
Rosette Formation Is Lost in Migrating Tg(hsΔTCF:GFP) Primordia (A) Rosettes can be visualized in 38hpf wt embryos by focal accumulation of Factin revealed by phalloidin staining (arrowheads). (B) eya1 in situ shows that wt primordia have completed migration and deposited all posterior neuromasts by 48hpf (L2-L4). (C) Heatshock induction of the dominant repressor ΔTCF:GFP at 28hpf leads to complete loss of rosettes by 38hpf as evidenced by phalloidin staining. At 28hpf the primordium is located just posterior to L3 (asterisk). (D) eya1 in situ at 48hpf shows that heatshocked Tg(hsΔTCF:GFP) primordia continue to migrate following the loss of rosettes. Activation of ΔTCF:GFP is more detrimental to the general health of the embryos than activation of dkk. Therefore, heatshocked Tg(hsΔTCF:GFP) primordia stall at around the position of L5 (arrow) and the embryos eventually die. |
|
In WT Embryos dkk1 Is Expressed Immediately Adjacent to the Leading Zone in which Wnt/β-Catenin Signaling Is Active Double in situ hybridization with dkk1 and the Wnt/β-catenin target lef1 at 32hpf reveals that Wnt/β-catenin signaling is not inhibited in cells immediately adjacent to dkk1 expressing cells. |
|
Sdf1a Signaling Is Not Necessary for Local Activation of the Wnt/β-Catenin Pathway in the Primordium In situ hybridization with axin2 reveals that activation of the Wnt/β-catenin pathway in the leading zone occurs normally in 26hpf sdf1a morphant primordia (A and B). The sdf1a morphant primordium has not started to migrate and is still posterior to the otic capsule. |
Reprinted from Developmental Cell, 15(5), Aman, A., and Piotrowski, T., Wnt/beta-catenin and Fgf signaling control collective cell migration by restricting chemokine receptor expression, 749-761, Copyright (2008) with permission from Elsevier. Full text @ Dev. Cell