- Title
-
Keratin 8/18a.1 Expression Influences Embryonic Neural Crest Cell Dynamics and Contributes to Postnatal Corneal Regeneration in Zebrafish
- Authors
- Williams, A.L., Bohnsack, B.L.
- Source
- Full text @ Cells
|
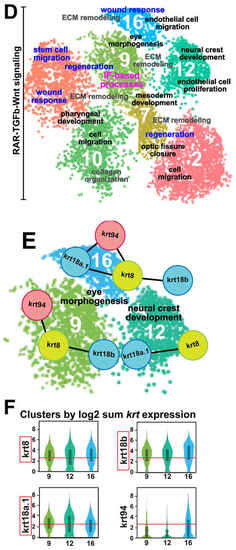
Keratin intermediate filament genes are expressed during early ocular development. (A) Step 1: Live images (lateral views) of the heads of 48-hpf Tg(-4.7sox10::EGFP) and Tg(foxd3::GFP) embryos for the collection of distinct sox10 (left panel) and foxd3 (right panel) POM neural crest cell subpopulations by FACS to isolate the GFP+ cells. Step 2: Following quality checks, the samples were loaded onto the 10× Genomics Chromium platform for the partitioning and encapsulation of single cells into nanoliter-sized GEMs (Gel beads-in-EMulsion). Each encapsulated cell was then lysed within its GEM, and the released RNA was reverse transcribed to cDNA with primers attached to a gel bead carrying a unique 10× barcode for downstream cell separation. Subsequently, the GEMs were broken, and all uniquely barcoded cDNAs were pooled, followed by PCR to generate enough material. Step 3: Illumina sequencing and library construction were performed using the 10× Genomics Single Cell 3′ v3.1 Protocol. Raw sequencing data were demultiplexed and the FASTQ files were aligned to the zebrafish reference genome (danRer11) using Cell Ranger. Loupe Browser (version 7.0.1) was used to evaluate the data and for further downstream analysis. (B) Cluster distribution at 48 hpf. Sox10:GFP and foxd3:GFP periocular neural crest cell subpopulations primarily clustered together, with few non-overlapping sox10+ and foxd3+ clusters (left panel). In total, 21 functional subgroups were observed in the combined dataset (right panel). (C) Cell type analysis based on the high expression of periocular mesenchyme (POM) transcription factors previously implicated in regulating ocular neural crest cell migration and differentiation (eya2, foxc1a, foxc1b, foxo1a, lmx1ba, lmx1bb, pitx2, tfap2a, and tfap2b), revealed 7 functional subgroups of interest. (D) Pathway enrichment analysis (Metascape) identified common and distinct putative biological functions (black and gray font) represented by the genes in the 7 functional subgroups of interest. In addition, healing and repair processes (blue font) were also highlighted amongst these functional subgroups. Moreover, the functional subgroup (Cluster 9) associated with ‘eye morphogenesis’, ‘ECM remodeling’, and ‘regeneration’ was also related to ‘intermediate filament (IF)-based processes’ (magenta font). (E) STRING analysis identified intermediate filament gene networks comprising krt94, krt8, krt18a.1, and krt18b among clusters associated with ‘eye morphogenesis’ and ‘neural crest development’ (Clusters 9, 12, 16). (F) Intermediate filament binding partners krt8 and krt18a.1/18b showed marked upregulated expression (at least 2.5x, as indicated by the red lines) during anterior segment development. EXPRESSION / LABELING:
|
|
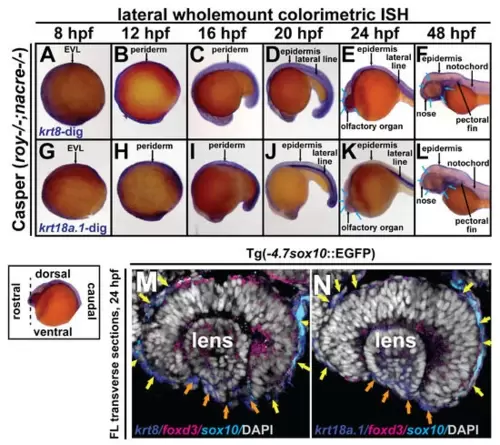
Krt8/krt18a.1 are expressed in the ocular and periocular neural crest during early development. Wholemount in situ hybridization in Casper (roy-/-; nacre-/-) zebrafish embryos during early development at 8, 12, 16, 20, 24, and 48 hpf. K8 and K18a.1 gene expression was detected using a colorimetric assay (Vector Blue Substrate Kit, Vector Laboratories) that is both chromogenic (blue) and fluorescent (Cy5). The sections were mounted in a media containing DAPI (gray). Lateral brightfield wholemount images show krt8 (A–F) and krt18a.1 (G–L) expression initiating dorsally along the neural plate border and enveloping layer (EVL) at 8 hpf (A,G) then dorsoposteriorally in the embryonic epithelium and ventrally in the ocular and craniofacial regions (B–E,H–K), with apparent expression in the ocular anterior segment and facial mesenchyme (blue arrows, (F,L)) by 48 hpf. Fluorescent double in situ hybridization for krt8 (M) or krt18a.1 (N) (Cy5/dark blue) and foxd3 (Texas Red/magenta) expression, followed by immunohistochemistry for sox10-GFP (α-GFP/light blue) was performed on transverse cephalic sections. The black dashed line (lower left insert) indicates the orientation of the plane of section, which passes perpendicular to the spinal column and extends in the rostral-caudal direction. Fluorescent confocal microscopy revealed krt8/krt18a.1 expression in the neural crest-derived ocular anterior segment (primordial cornea, orange arrows) and periocular mesenchyme (yellow arrows) of zebrafish embryos at 24 hpf. EXPRESSION / LABELING:
|
|
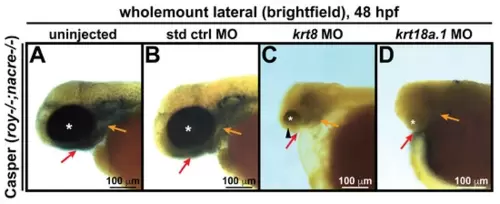
Krt8/Krt18a.1 knockdown disrupts ocular and craniofacial development. Wholemount imaging showed that compared with their uninjected (A) and standard control (std ctrl) MO-injected (B) counterparts, krt8 MO-injected zebrafish embryos (C) developed microphthalmic eyes (asterisk) with coloboma (arrowhead), while krt18a.1 MO-injected embryos (D) had severely underdeveloped or anophthalmic eyes (asterisk) at 48 hpf, suggesting that the effects were more severe with Krt18a.1 knockdown. The absence of pharyngeal arch (orange arrows) and jaw (red arrows) formation was observed after MO knockdown in both cases. PHENOTYPE:
|
|
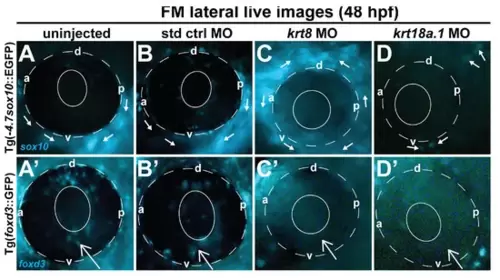
Krt8/Krt18a.1 MO knockdown disrupted ocular neural crest cell migration dynamics during early eye development. Lateral live imaging at 48 hpf shows the effects of Krt8 and Krt18a.1 knockdown on GFP reporter expression in Tg(foxd3::EGFP) and Tg(-4.7sox10::EGFP) zebrafish embryos injected at the single-cell stage with antisense MOs targeting krt8 and krt18a.1. (A,A′) uninjected, (B,B′) standard control (std ctrl) MO-injected, (C,C′) krt8 MO-injected, and (D,D′) krt18a.1 MO-injected. The migration of GFP-positive neural crest cells into the ocular (open arrow) and periocular (solid arrows) regions was markedly disrupted in response to Krt8/Krt18a.1 MO knockdown compared with that in uninjected and control MO-injected embryos. The solid and dashed circles highlight the lens and retinal pigment epithelium, respectively, of the zebrafish eye. d, dorsal; v, ventral; p, posterior; a, anterior. PHENOTYPE:
|
|
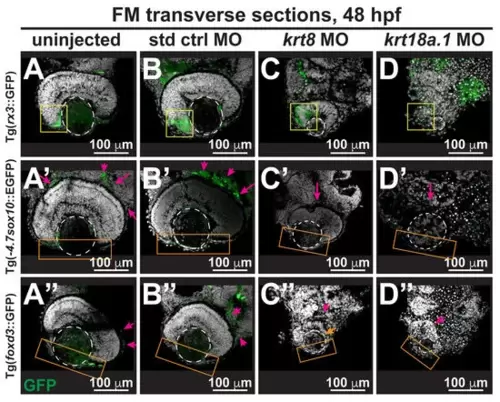
Krt8/Krt18a.1 play roles in neural crest migration and patterning during early eye development. Section analysis of 48-hpf Tg(-4.7sox10::EGFP), Tg(foxd3::GFP), and Tg(rx3::GFP) zebrafish embryos showed that MO knockdown of K8 or K18a.1 resulted in the significant disorganization of neural crest cells in the optic cup rim (yellow box, (C,D)), periocular mesenchyme (magenta arrow, (C′,D′,C″,D″)), and ocular anterior segment (orange box, (C′,D′,C″,D″)). The orange arrow indicates slight foxd3 signal in the anterior segment of the underdeveloped eye. The dashed circles highlight the lens. (E) Quantitative analysis showed a significant decrease of rx3, sox10, and foxd3 promoter-driven GFP expression in the periocular and ocular regions of the developing eyes of krt8/krt18a.1 morphants compared with those of uninjected (A–A″) and control MO-injected fish (B–B″). Notably, these effects were more severe with K18a.1 knockdown. **, p-value < 0.01; n.s., not significant. PHENOTYPE:
|
|
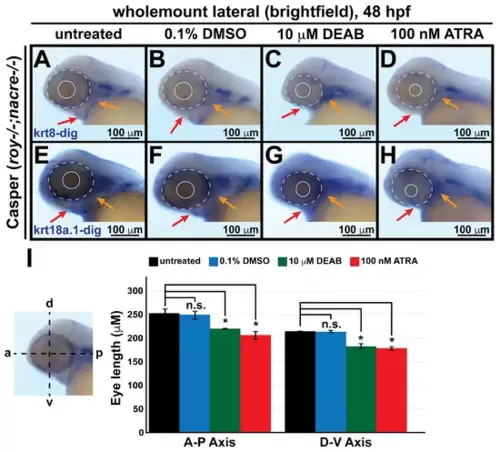
Changes in retinoic acid levels affect early ocular and craniofacial development. Wholemount in situ hybridization was performed using a chromogenic (blue) colorimetric assay. Lateral images (A–D,E–H) of treated zebrafish embryos taken at 48 hpf show the teratogenic effects of pharmacological insult with 10 μM DEAB and 100 nM ATRA on the ocular and craniofacial neural crest during early development. The solid and dashed circles highlight the reduced eye size. The red and orange arrows highlight jaw and pharyngeal arch malformation, respectively. (I) Quantitative analysis of this effect shows that treatment with both DEAB and ATRA delayed ocular development and significantly decreased the eye size of the treated fish, as measured along the anterior–posterior (a-p) and dorsal-ventral (d-v) axes. *, p-value < 0.05; n.s., not significant. PHENOTYPE:
|
|
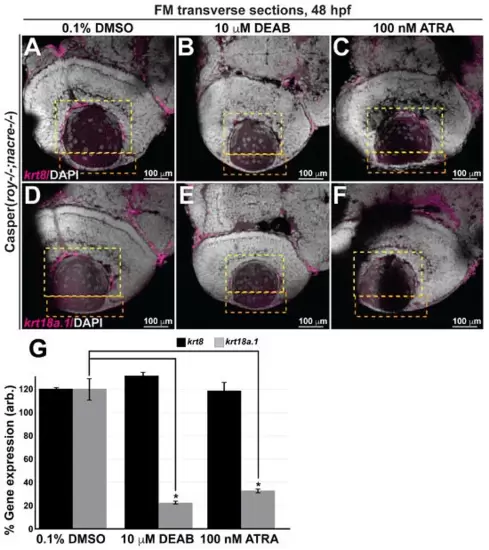
Retinoic acid regulates krt18a.1, but not krt8, expression in the eye during early embryogenesis. (A–F) Treatment of Casper zebrafish embryos at 24 and 27 hpf with DEAB and RA, respectively, followed by wholemount colorimetric in situ analysis shows decreased krt18a.1, but not krt8, expression in the developing eye in response to pharmacological insult. (G) Quantification of these effects, as measured in the anterior chamber (orange dashed box) and hyaloid (yellow dashed box) regions of the eyes of 48-hpf zebrafish, further shows that compared with the DMSO vehicle control, treatment with either DEAB or ATRA significantly decreased krt18a.1 expression, while that of krt8 was not affected. *, p-value < 0.05. |
|
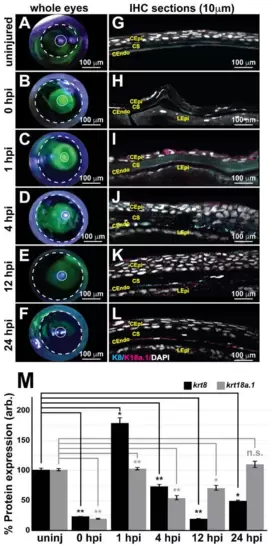
Keratin intermediate filament proteins 8 and 18a.1 are expressed at various time points during postnatal corneal wound healing. A zebrafish adult ocular injury model was established to achieve extensive corneal injury consisting of epithelial debridement, removal of Bowman’s membrane, and excavation of the anterior to mid stroma. (A–F) Fluorescein tracing to distinguish injured corneal surfaces shows the complete healing of the injured eye by 24 h post injury (hpi). The dashed and solid circles highlight the iris and pupil, respectively, in the zebrafish eye. (G–L) Immunohistochemical analysis of thin (10 μm) sections shows that the corneal stroma regenerated within 1 h following mechanical injury, and the expression of both K8 and K8a.1 in neural crest-derived tissues (stroma and endothelium) was detected in the uninjured (uninj) cornea and during ocular wound healing. CEpi, corneal epithelium; CS, corneal stroma; CEndo, corneal endothelium. (M) Quantitative analysis of K8 and K18a.1 expression shows differential expression patterns during corneal wound healing. *, p-value < 0.05; **, p-value < 0.01; n.s., not significant. |
|
RA influences injury repair and regulates K18a.1 expression in the postnatal neural crest. Adult zebrafish subjected to ocular injury were subsequently recovered in system water containing lidocaine (2.5 mg/L) or lidocaine in combination with either 0.1% DMSO, 10 mM DEAB, or 100 nM ATRA. (A–F) Fluorescein tracing shows that corneal healing was remarkably disrupted by treatment with DEAB (E) and ATRA (F). The dashed and solid circles highlight the iris and pupil, respectively, in the zebrafish eye. (G–L) Cryosection (10 μm), followed by immunohistochemical analysis revealed significant stromal edema (K) and epithelial (L) edema in response to alterations in RA levels during ocular wound healing. CEpi, corneal epithelium; CS, corneal stroma; CEndo, corneal endothelium. (M,N) Quantitative analysis of K8 and K18a.1 expression revealed decreased Krt18a.1 expression in response to pharmacological insult during corneal wound healing. *, p-value < 0.05; **, p-value < 0.01; ***, p-value < 0.001; n.s., not significant. |