- Title
-
Rspo1/Wnt signaling promotes angiogenesis via Vegfc/Vegfr3
- Authors
- Gore, A.V., Swift, M.R., Cha, Y.R., Lo, B., McKinney, M.C., Li, W., Castranova, D., Davis, A., Mukouyama, Y.S., and Weinstein, B.M.
- Source
- Full text @ Development
|
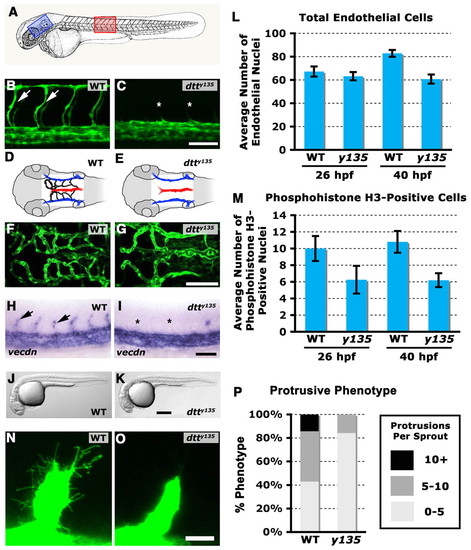
dtty135 mutants have defects in angiogenesis, but not in endothelial specification or in vasculogenesis. (A) Diagram of a zebrafish embryo with the red box highlighting the region shown in B and C, and a blue box highlighting the region shown in D-G. (B,C) Confocal images of trunk vessels in 26 hpf Tg(fli-EGFP)y1 wild-type sibling (B) and dtty135 mutant (C) zebrafish, showing normal formation of the vasculogenic dorsal aorta and posterior cardinal vein but failure to form the angiogenic intersegmental vessels (arrows in B, asterisks show absence in C) in dtty135 mutants (lateral views, anterior towards the left). (D,E) The hindbrain vessels imaged in F,G. Primordial hindbrain channels (PHBC) are in blue, basilar artery (BA) is in red and the central arteries (CA) are in black. (F,G) Confocal images of hindbrain vessels in 48 hpf Tg(fli-EGFP)y1 wild-type sibling (F) and dtty135 mutant (G) zebrafish, showing normal formation of vasculogenic primary vessels, including the PHBC and BA, but failure to form the angiogenic CA that penetrate the hindbrain (dorsal views, anterior towards the left). (H,I) In situ hybridization of endothelium in the trunk of 26 hpf wild-type sibling (H) and dtty135 mutant (I) zebrafish with a probe for vecdn, showing normal expression levels in dtty135 mutants (lateral views, anterior towards the left). ISV are seen in wild-type siblings (arrows in H) but not in dtty135 mutants (asterisks in I). (J,K) Transmitted light images of 26 hpf Tg(fli-EGFP)y1 wild-type sibling (J) and dtty135 mutant (K) zebrafish, showing reduced width of yolk extension but otherwise normal morphology of dtty135 mutants (J) compared with their wild-type siblings (K). Images are lateral views, anterior towards the left. Endothelial proliferation is reduced in dtty135 mutants. (L) Total number of endothelial cells present in the three posteriormost trunk segments, measured in Tg(fli-nEGFP)y7 transgenic dtty135 mutant or wild-type sibling animals, at 26 hpf and 40 hpf. (M) Total number of phosphohistone H3-positive endothelial cells present in the three posteriormost trunk segments, measured in phosphohistone and GFP antibody-probed Tg(fli-nEGFP)y7 transgenic dtty135 mutant or wild-type sibling animals, at 26 hpf and 40 hpf. Data are meanąs.e.m. (N,O) Higher-magnification confocal images of ISV sprouts in wild-type sibling (N) and dtty135 mutant (O) embryos at 20 hpf, showing reduced filopodial protrusions in mutants. (P) Total number of filopodia formed by control and mutant sprouts. Scale bars: 50 μm in B,C,F,G; 100 μm in H-K; 20 μm in N,O. PHENOTYPE:
|
|
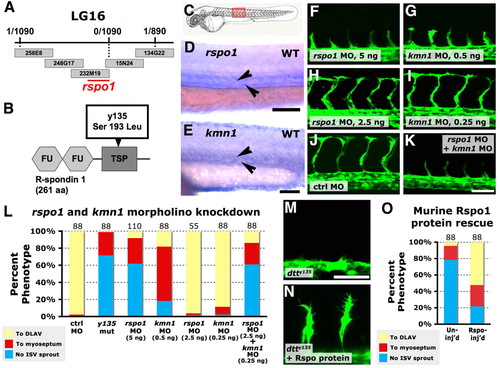
R-spondin 1 and its receptor kremen 1 are required together for angiogenesis. (A) dtty135 genetic interval on linkage group 16, with the number of recombinants per meiosis, position of BAC clones and position of the rspo1 locus indicated. (B) Rspo1 protein domain structure, with two furin domains (FU) and a thrombospondin domain (TSP). The dtty135 mutation changes serine 193 to leucine in the TSP domain. (C) A zebrafish embryo with the red box highlighting the region imaged in D-K. (D,E) In situ hybridization of the trunk of 24 hpf wild-type zebrafish embryos probed for rspo1 (D) or krm1 (E), with expression observed in the axial vasculature (arrowheads). (F-K) Confocal images of trunk vessels in 26 hpf Tg(fli-EGFP)y1 wild-type zebrafish injected with either 5 ng rspo1 MO (F), 0.5 ng krm1 MO (G), 2.5 ng rspo1 MO (H), 0.25 ng krm1 MO (I), 5 ng control MO (J) or 2.5 ng rspo1 MO + 0.25 ng krm1 MO (K). (L) Quantitation of the intersegmental vessel (ISV) phenotypes of 26 hpf Tg(fli-EGFP)y1 dtty135 mutant or morpholino-injected embryos. (M,N) Confocal images of ISV in 26 hpf Tg(fli-EGFP)y1 dtty135 mutant embryos that were either not injected (M) or were injected intramuscularly in the trunk with murine R-spondin (N). (O) Quantitation of the intersegmental vessel (ISV) phenotypes of uninjected or mouse R-spondin-injected dtty135 zebrafish at 28 hpf. In L and O the bars show the percentages of ISV that have failed to sprout (blue), ISV that have grown only up to the horizontal myoseptum half way up the trunk (red) and ISV that have grown all the way to the dorsal trunk to form the DLAV (yellow). The number of segments counted is shown above each bar on the graphs. Scale bars: 100 μm in D; 50 μm in E; 50 μm in F-K,M,N. |
|
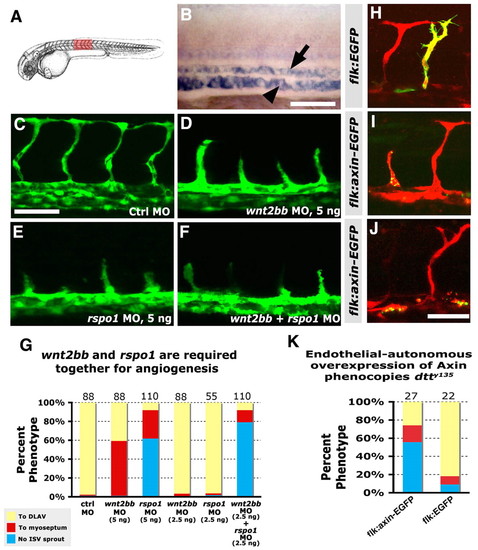
Canonical Wnt signaling is required endothelial cell-autonomously for angiogenesis. (A) Diagram of a zebrafish embryo with the red box highlighting the region shown in B-F,H-J. (B) In situ hybridization of the trunk of a 26 hpf wild-type zebrafish embryo probed for wnt2bb, showing expression in the axial vessels (dorsal aorta, arrow; posterior cardinal vein, arrowhead). Lateral view, anterior towards the left. (C-F) Confocal images of trunk vessels in 26 hpf Tg(fli-EGFP)y1 wild-type zebrafish injected with either 5 ng control MO (C), 5 ng wnt2bb MO (D), 5 ng rspo1 MO (E) or 2.5 ng wnt2bb MO + 2.5 ng rspo1 MO (F). (G) Quantitation of the intersegmental vessel (ISV) phenotypes of morpholino-injected wild-type 26 hpf Tg(fli-EGFP)y1 zebrafish. (H-J) Confocal images of trunk vessels in 26 hpf wild-type Tg(flk-mCherry) zebrafish (red) injected with DNA for either control flk:EGFP (H) or flk:axin-EGFP (I,J) expression constructs (green), showing defects in sprouting (J) or growth (I) of ISV expressing the axin-EGFP fusion protein. (K) Quantitation of the intersegmental vessel (ISV) phenotypes of endothelium expressing flk:axin-EGFP or control flk:EGFP expression constructs. In G and K bars show the percentages of ISV that have failed to sprout (blue), ISV that have grown only up to the horizontal myoseptum half-way up the trunk (red) and ISV that have grown all the way to the dorsal trunk to form the DLAV (yellow). The number of segments counted is shown above each bar. Scale bars: 50 μm in B-F,H-J. |
|
Upregulation of the Wnt/β-catenin pathway promotes angiogenesis. (A,B) Confocal images of trunk vessels in 48 hpf Rspo1 MO-injected wild-type (+/+) or heterozygous (apcmcr/+) embryo with ISV defects (A) or homozygous (apcmcr/apcmcr) mutant (B) with relatively normal ISV growth. (C) Quantitation of ISV phenotype of Rspo1 MO-injected wild type (+/+) or heterozygous (apcmcr/+) embryo and homozygous (apcmcr/apcmcr) mutant. (D,E) In situ hybridization of the trunks of 26 hpf wild-type zebrafish embryos treated from 16 hpf to 24 hpf with either carrier DMSO (D) or BIO (E), probed for cyclin D1. (F,G) In situ hybridization of the trunks of 6 hpf wild-type zebrafish embryos treated from 2.5 hpf to 6 hpf with either carrier DMSO (F) or BIO (G), probed for chordin. (H,I) Confocal images of hindbrain vessels in 48 hpf Rspo1 MO-injected embryos treated with carrier DMSO (H) or BIO (I). (J,K) Confocal images of trunk vessels in 40 hpf dtty135 mutant embryos treated with carrier DMSO (J) or BIO (K) from 16 hpf. (L) Quantitation of the intersegmental vessel (ISV) phenotypes of 40 hpf Tg(fli:EGFP)y1 dtty135 mutants treated from 16-40 hpf with either DMSO carrier or BIO. Bars show the percentages of ISV that have failed to sprout (blue), ISV that have grown only up to the horizontal myoseptum half-way up the trunk (red) and ISV that have grown all the way to the dorsal trunk to form the DLAV (yellow). The number of segments counted is shown above each bar. Scale bars: 50 μm in A,H,J. |
|
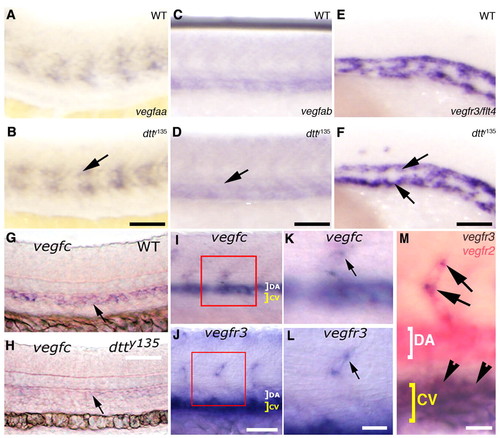
Expression of Vegfc is specifically affected in dtty135 mutant embryos. (A-F) In situ hybridization of the mid-trunk of wild-type sibling (A,C,E) and dtty135 mutant (B,D,F) zebrafish embryos probed for vegfaa (A,B), vegfab (C,D) or vegfr3 (E,F). The expression of vegfaa in the somites (arrow in B), vegfab in the axial vessels (arrow in D) and vegfr3 in the axial vessels (arrows in F) does not change in dtty135 mutant embryos compared with their wild-type siblings. (A-D) 24 hpf; (E,F) 20 hpf. (G,H) In situ hybridization of 26 hpf wild-type sibling (G) and dtty135 mutant (H) embryos probed for vegfc (arrows). (I,K) In situ hybridization of 26 hpf wild-type embryos probed for vegfc, showing expression in the DA and ISV (K shows a magnified image of the boxed region in I). (J,L) In situ hybridization of 26 hpf wild-type embryos probed for vegfr3, showing expression in the CV and ISV tip cells (L shows a magnified image of the boxed region shown in J). (M) Double in situ hybridization confirming expression of Vegfr3/flt4 (purple) in the tip cell (arrows) in addition to the cardinal vein (arrowheads), when compared with expression of Vegfr2/flk1 (pink) throughout the DA, CV and ISV. Scale bars: 50 μm in B,D,F,G; 45 μm in I,J; 25 μm in K-M. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Expression of vegfc is specifically regulated by Wnt/β-catenin signaling. (A-D) In situ hybridization of the mid-trunk of 24 hpf of wild-type sibling (A,C), etsrpy11 mutant (B) and flky17 mutant (D) embryos, probing for vegfc. Arrows show vegfc expression in the dorsal aorta, which is not affected in the two mutants. (E,F) RT-PCR analysis showing expression of vegfc by cultured mouse endothelial cells upon treatment with BIO (E) or Wnt3a (F). (G,H) In situ hybridization of 26 hpf rspo1 MO-injected embryos treated from 16-26 hpf with either 0.5% DMSO carrier (G) or 0.5 μM BIO in DMSO (H), probed for vegfc (arrows). (I,J) In situ hybridization of 36 hpf wild-type sibling (I) or homozygous (apcmcr/apcmcr) mutant (J) embryos probed for vegfc (arrows). Scale bars: 50 μm. |
|
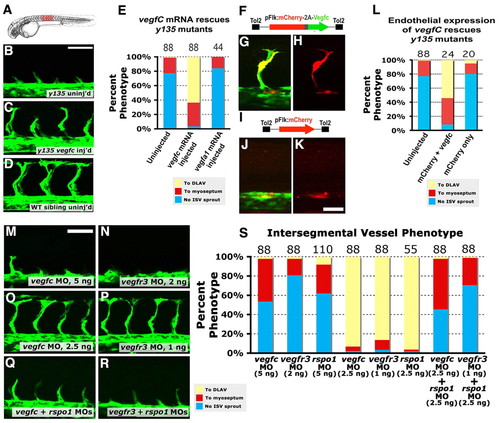
Vegfc signaling is required downstream of Rspo/Wnt signaling. (A) Diagram of a zebrafish embryo with the red box highlighting the region shown in B-D. (B-D) Confocal images of the trunk vessels in 26 hpf dtty135 mutant (B), vegfc mRNA-injected dtty135 mutant (C) and uninjected wild-type sibling (D). (E) Quantitation of ISV growth phenotypes in 26 hpf uninjected and mRNA-injected zebrafish embryos. P values according to Student?s t-test: vegfc, P<0.0001; vegfa, P<0.0187. (F-H) Rescued ISV sprouts in 26 hpf Tg(fli-EGFP)y1 dtty135 mutant zebrafish injected with a Tol2(Flk:mCherry-2a-vegfc) construct (F) for Flk promoter-driven endothelial expression of mCherry and vegfC separated by a viral 2A peptide sequence. (I-K) No rescue of ISV sprouting in 26 hpf Tg(fli-EGFP)y1 dtty135 mutant zebrafish injected with a Tol2(Flk:mCherry) Tol2 construct (I) for Flk promoter-driven endothelial expression of mCherry reporter alone. (G,J) EGFP (green) and mCherry (red) combined fluorescence image. (H,K) Same fields as in G,J, viewing mCherry fluorescence alone. (L) Quantitation of ISV growth phenotypes in 26 hpf uninjected and Tol2(Flk:mCherry-2a-vegfc) or Tol2(Flk:mCherry) DNA-injected zebrafish embryos. The bars show the percentages of ISV that have failed to sprout (blue), ISV that have grown only up to the horizontal myoseptum half-way up the trunk (red) and ISV that have grown all the way to the dorsal trunk to form the DLAV (yellow). For DNA construct-injected animals in L, only mCherry-positive ISV phenotypes were assessed. The number of segments counted is shown above each bar on the graphs. (M-R) Confocal images of trunk vessels in 26 hpf Tg(fli-EGFP)y1 wild-type zebrafish injected with either 5 ng vegfc MO (M), 2 ng vegfr3 MO (N), 2.5 ng vegfc MO (O), 1 ng vegfr3 MO (P), 2.5 ng vegfc + 2.5 ng rspo1 MO (Q) or 1 ng vegfr3 MO + 2.5 ng rspo1 MO (R). (S) Quantitation of ISV growth phenotypes in 26 hpf different MO-injected zebrafish embryos. Color coding is the same as in L. Scale bars: 50 μm in B,M; 25 μm in K. |
|
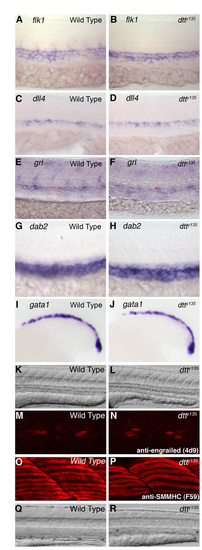
Endothelial, hematopoietic and muscle patterning is normal in dtty135 mutants. (A-J) In situ hybridization of the trunks of wild-type (A,C,E,G,I) and dtty135 mutant (B,D,F,H,J) zebrafish embryos probed for flk1 (A,B), dll4 (C,D), grl (E,F), dab2 (G,H) and gata1 (I,J). Marker expression appears equivalent in wild-type and dtty135 mutant animals. Embryos are at 26 hpf (A-H) and 18 hpf (I,J). (K,L) DIC transmitted light images of the trunks of 26 hpf wild-type (K) and dtty135 mutant (L) animals, showing normally patterned somites and muscles in dtty135 mutants. (M,N) Confocal imaging of trunks of wild-type (M) and dtty135 mutant (N) animals stained for immunofluorescence with a monoclonal anti-engrailed antibody (4D9), revealing normal muscle pioneer specification. (O,P) Confocal imaging of trunks of wild-type (O) and dtty135 mutant (P) animals stained for immunofluorescence with a monoclonal anti-SMMHC antibody (F59), revealing normal slow muscle differentiation. (Q,R) DIC transmitted light images of the trunks of 96 hpf wild-type (Q) and dtty135 mutant (R) animals, showing reduced muscle mass by 96 hpf in dtty135 mutants. Lateral views, anterior towards the left. EXPRESSION / LABELING:
|
|
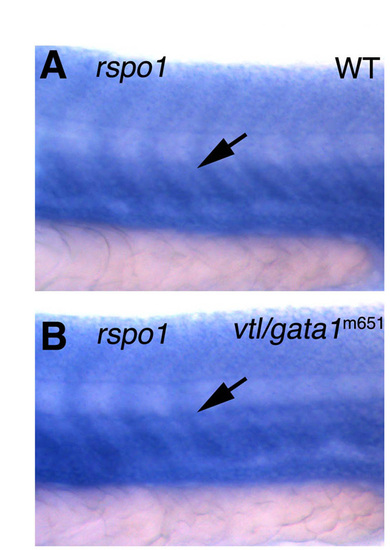
Expression of rspo1 is not affected in vlt/gata1<b>m651 mutant embryos. In situ hybridization of the mid-trunk of 24 hpf of wild-type sibling (A) and a vlt/gata1m651 mutant (B) embryos, probing for rspo1. Arrows show rspo1 expression in the trunk vasculature, which is not affected in the two mutants. EXPRESSION / LABELING:
|
|
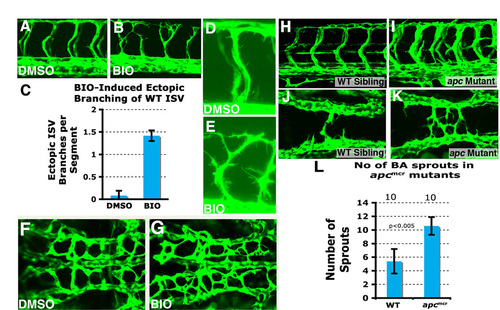
Ectopic vascular branching in BIO treated wild-type and Rspo1 morphant embryos. (A,B) Confocal images of the trunks of 40 hpf wild-type Tg(fli:EGFP)y1 zebrafish embryos treated from 16 hpf to 40 hpf with either carrier DMSO (A) or BIO (B). BIO-treated animals show enhanced ISV branching compared with their control DMSO-treated siblings. (C) Quantification of the number of ectopic branches per segment in trunks of 40 hpf wild-type Tg(fli:EGFP)y1 zebrafish embryos treated from 16 hpf to 40 hpf with either carrier DMSO or BIO. (D,E) Higher magnification confocal images of the trunks of 40 hpf wild-type Tg(fli:EGFP)y1 zebrafish embryos treated from 16 hpf to 40 hpf with either carrier DMSO (D) or BIO (E), showing increased protrusive activity in BIO-treated embryos compared with DMSO-treated controls. (F,G) Confocal images of the central arteries of 48 hpf Tg(fli:EGFP)y1 wild-type zebrafish embryos treated with DMSO (F) or BIO (G). (H,I) Confocal images of the trunk vessels of 48 hpf wild-type sibling (H) and homozygous apcmcr (I) mutant embryos. (J,K) Confocal images of the hindbrain cranial vessels of 30 hpf wild-type sibling (J) and homozygous apcmcr (K) mutant embryos. (L) Quantitation of number of basilar artery sprouts at 30 hpf in wild-type sibling and homozygous apcmcr mutant embryos. |
|
Rspo1-Wnt-vegfc signaling does not interact with Notch signaling. Vascular Notch signaling is not affected in rspo1 MO-injected animals. (A,B) Expression of mCherry in the mid-trunk of 24 hpf Tg(T2KTp1bglob:hmgb1-mCherry)jh11 transgenic Notch reporter embryos (Parsons et al., 2009) injected with either control MO (A) or rspo1 MO (B). Notch-dependent expression of mCherry in the vasculature (arrows) is unchanged in rspo1 MO- compared with control MO-injected animals. The expression of vegfc is not affected by ubiquitous heatshock-inducible transgenic activation of Notch signaling. (C,D) In situ hybridization of the mid-trunk of 24 hpf wild-type sibling embryos (C) or Tg(hsp70:Gal4)1.5kca4, Tg(UAS:myc-notch1a-intra)kca3 double transgenic embryos for heatshock-inducible activation of Notch signaling (D), as described previously by Lawson et al. (Lawson et al., 2001). Vascular expression of vegfc (arrows) is unchanged in Notch-activated embryos when compared with their control siblings. Lateral views, anterior towards the left. |