- Title
-
Mutation of the zebrafish nucleoporin elys sensitizes tissue progenitors to replication stress
- Authors
- Davuluri, G., Gong, W., Yusuff, S., Lorent, K., Muthumani, M., Dolan, A.C., and Pack, M.
- Source
- Full text @ PLoS Genet.
|
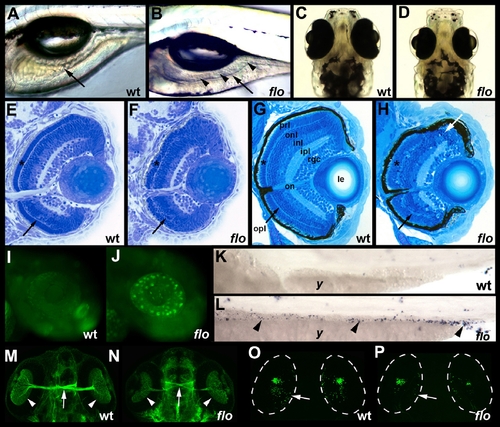
flo intestinal and retinal defects. (A,B) Lateral view of live 5 dpf wild type (wt) and flo larvae. The flo intestine lacks folds (arrow) and the lumen contains detached epithelial cells (arrowheads). (C,D) Dorsal view showing reduced size of the 5 dpf flo eye. (E,F) Histological cross section showing cells with condensed nuclei typical of apoptotic cells in the 60 hpf flo retina, and disorganization of the flo photoreceptor (*) and outer plexiform (arrow) layers. (G,H) Histological cross section showing marked disorganization of the 4 dpf flo retina and cells with condensed nuclei typical of apoptotic cells (white arrow). (I,J) Acridine orange staining showing apoptotic cells in the 48 hpf flo retina but not sibling wild types. (K,L) TUNEL staining showing apoptotic cells in the 75 hpf flo intestine (arrowheads) but not sibling wild types (anterior?left, posterior?right). (M,N) Dorsal view showing mild reduction in the number of flo retinal ganglion cells (arrowheads) and optic nerve diameter (arrow) identified with the Zn5 antibody. (O,P) Confocal projection of immunostained wt and flo larvae showing reduced rod cells in the flo retina including the large ventral cluster of cells and in the periphery of the mid retina (arrow). onl, outer nuclear layer; inl, inner nuclear layer; ipl, inner plexiform layer; rgc, retinal ganglion cell layer; on, optic nerve; le, lens; y, yolk. |
|
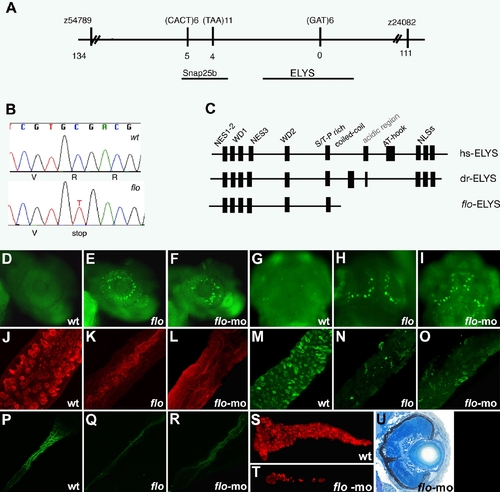
The flo locus encodes zebrafish elys. (A) Schematic representation of the genomic region surrounding the flo locus. The names of the polymorphic markers with the corresponding number of recombinants are listed. (B) DNA sequence analysis showing the cytosine to thymidine transition encoding the premature stop codon in the elysti262c allele. (C) Schematic representation of the functional domains of the human (hs) and zebrafish (dr) Elys protein and the protein encoded by the elysti262c allele (flo-ELYS). (D?I) Acridine orange staining showing apoptotic cells in the retina and growth plate of the optic tectum of 48 hpf flo (E,H) and elys-morpholino injected (F,I) larvae but not wt (D,G). (J?R) Confocal projections through the posterior intestine of 96 hpf larvae showing wheat-germ agglutinin positive goblet cells in the epithelium of the posterior intestine of wt (J) but not flo (K) or elys-morpholino injected (L) larvae; secretory cells in wt (M) but not flo (N) or elys-morpholino (O) injected larvae; enterocytes in wt (P) but not flo (Q) or elys-morpholino (R) injected larvae. (S?T) Carboxy-peptidase A positive cells are abundant in the 5 dpf wt (S) but not in elys-morpholino injected (T) exocrine pancreas. (U) Histological cross section through the retina of a 4 dpf elys morpholino injected larva showing retinal disorganization that is comparable to the 4 dpf flo retina (Figure 1H). |
|
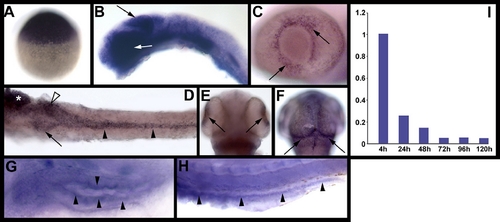
elys expression in developing zebrafish. Images (A?H) are whole mount RNA in situ hybridizations. (A) Maternal elys expression at 3 hpf. (B) Strong elys expression is evident in the midbrain (black arrow) and eye (white arrow) at 24 hpf. (C?F) Expression at 48 hpf in the retina (C,E), growth plate of the optic tectum (F), and digestive organs [(D): pancreas (arrow), anterior intestine (white arrowhead), posterior intestine (black arrowheads), liver (*)]. (G?H) Weak elys expression in the 5 dpf anterior (G) and posterior (H) intestine. (I) Graph showing elys expression in whole embryos (4 hpf?120 hpf) as determined via real-time quantitative PCR. |
|
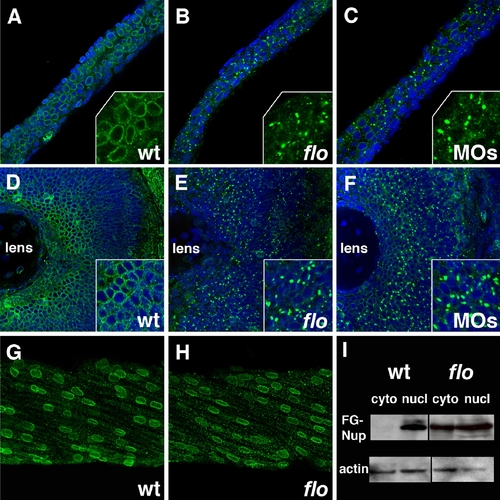
Nuclear pore disruption in flo mutants. (A?C) Confocal projections through the posterior intestine of 75 hpf wild type (A), flo (B), and elys morpholino injected larvae (C), following anti-FG nucleoporin immunostainings with mAb414 (green; DAPI?blue). There is a dramatic reduction of nuclear pores in the flo and morpholino injected larvae. Inset shows higher magnification of localized regions of the DAPI-stained image. (D?F) Identical findings are evident in the retina of these larvae. Note apparent cytoplasmic accumulation of the immunoreactive FG-nucleoporins in flo and morpholino injected larvae. (G,H) Normal nuclear distribution of FG nucleoporins in wild type and flo skeletal muscle. (I) Western blot showing levels of FG nucleoporin proteins relative to beta-actin in nuclear (nucl) and cytoplasmic (cyto) extracts derived from the intestine of 75 hpf flo and sibling wild type larvae. EXPRESSION / LABELING:
PHENOTYPE:
|
|
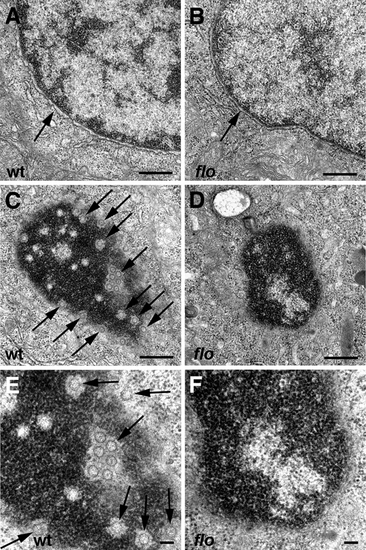
Nuclear ultrastructure in flo mutants. Transmission electron micrographs of nuclei from representative 5 dpf wild type and flo intestinal epithelial cells. (A,B) Intact nuclear envelope in wild type (A) and flo (B). (C?F) Tangential sections through the nuclear envelope showing abundant nuclear pores (arrows) in the wild type larva (C,E) but few if any well defined pores in the flo larva (D,F). (E) and (F) are higher magnification views of (C) and (D), respectively. PHENOTYPE:
|
|
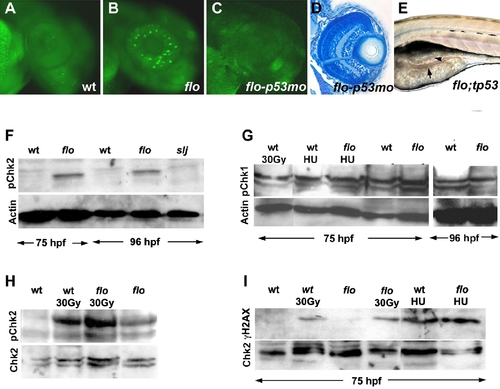
The flo mutation activates the DNA damage response. (A?C) Acridine orange staining showing apoptosis in the 50 hpf flo retina that is rescued by the tp53 morpholino (mo) knockdown. (D) Histological cross section showing rescue of flo retinal architecture defects by tp53 knockdown [compare (F) with Figure 1G and 1H]. (E) Intestinal defects persist in flo/tp53 double mutants. Arrow, thin intestinal wall; Arrowhead, apoptotic cells in the intestinal lumen. (F) Western blot showing elevated levels of phospho-Chk2 (Serine 33) in the intestine of flo larvae, compared with sibling wild type larvae, but not slim jim larvae (I). (G) Western blot showing comparable levels of phospho-Chk1 (Ser 345) in flo and sibling wild type larvae, before and after γ-radiation (30 Gy) and treatment with hydroxyurea (HU). (H) Western blot showing enhanced phospho-Chk2 activation in the intestine of flo and wild type larvae following γ-radiation (30 Gy). (I) γH2AX is not detected in the flo or wild type intestine (75 hpf), but is detected at this stage following γ-irradiation (30 Gy) or hydroxyurea treatment (HU). EXPRESSION / LABELING:
|
|
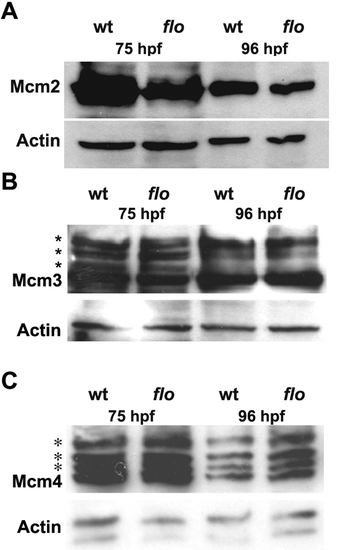
Reduced chromatin bound Mcm2 in the flo intestine. (A) Western analysis showing reduced levels of chromatin bound Mcm2 in 75 hpf and 96 hpf flo intestine compared with wild type siblings. By contrast, levels of chromatin bound Mcm3 (B) and Mcm4 (C) in the flo intestine are comparable to wild type. Multiple bands (*) corresponding to phospho-Mcm3 and phospho-Mcm4 are recognized by the antibodies directed against the native proteins in wt and flo samples. EXPRESSION / LABELING:
PHENOTYPE:
|
|
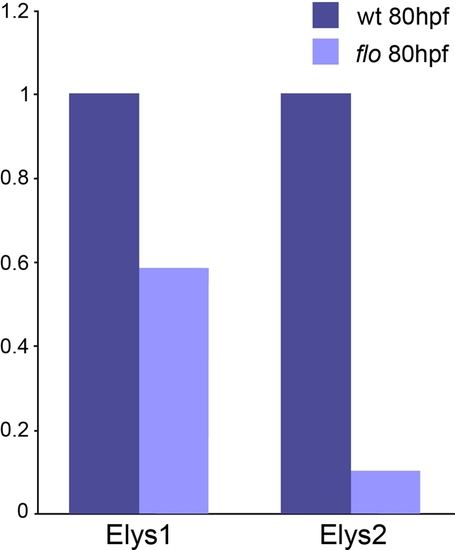
Reduced elys expression in flo larvae. Results from real time quantitative PCR amplification of elys cDNA fragments. These data show reduced elys expression in 5 dpf flo vs. sibling wild type larvae. The Elys-1 primers are located in exons 5/6. The Elys-2 primers are located in exons 22/23. The flo mutation is located in elys exon 30. Reduced elys expression in flo larvae is consistent with non-sense codon initiated mRNA decay induced by the floti262c mutation (23). EXPRESSION / LABELING:
|
|
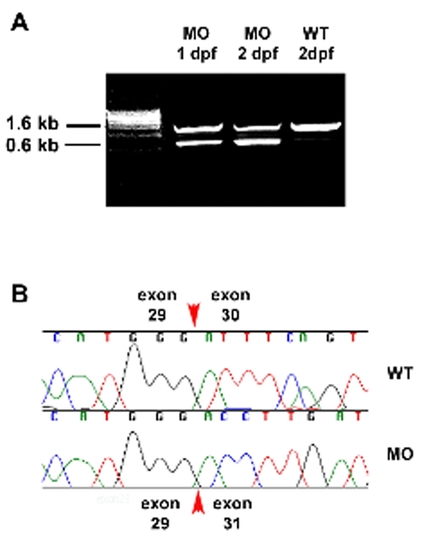
Morpholino induced elys cDNA truncation. (A) Ethidium bromide stained agarose gel showing 1 kb truncation of an elys cDNA fragment amplified from 1 dpf and 2 dpf wild type embryos that had been injected with the elys exon 30 splice junction morpholino. This morpholino targets genomic sequence at the intron 29/exon 30 splice acceptor. Successful targeting induces deletion of exon 30, as revealed by sequence analysis (B) of the 0.6 kb fragment. |
|
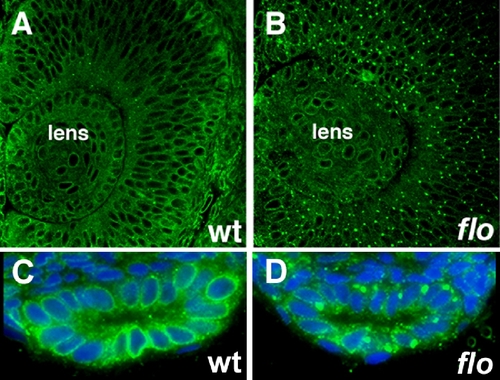
Nuclear pore defects in early flo mutants. (A-D) Histological sections of flo mutants and wild type siblings immunostained with anti-FG Nup antibody (mAb414) showing nuclear pore defects in the 36 hpf flo retina (A,B) and 48 hpf intestine (C,D). Blue, Dapi stained nuclei. |
|
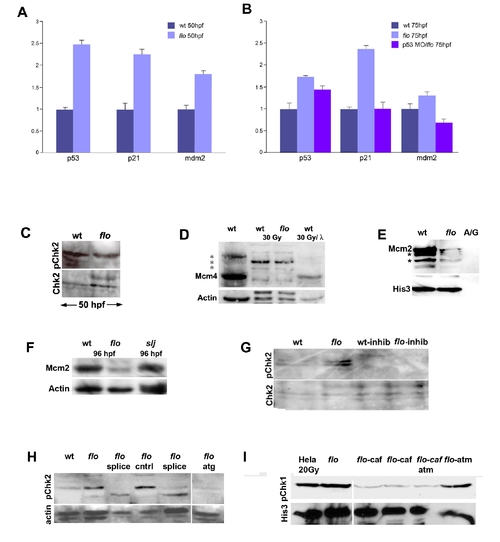
DNA damage response activation in flo mutants. (A,B) Quantitative PCR reveals increased p53, p21, and mdm2 expression in flo mutants. Note that tp53 knockdown in flo (B) abrogates increased p21 and mdm2 expression. (C) Western blot showing comparable levels of phospho-Chk2 in the flo and wild type eye (48 hpf). (D) Western blot showing native and phospho-Mcm4 (*) in the zebrafish wild type (wt), flo intestines before and after γ-irradiation (30 Gy). Note that there is very little native Mcm4 in wt and flo following γ-irradiation (lanes 2 and 3). Phosphatase treatment (λ) of the wt sample from lane 2 dephosphorylates nearly all of the phospho-Mcm4 protein such that only native Mcm4 is present in the sample. (E) Confirmatory Western blot showing reduced chromatin bound Mcm2 in the intestine of 84 hpf flo larvae compared with sibling wt larvae. Far right lane labeled ?A/G? shows undetectable levels of Mcm2 and Histone 3 recovered from Ig fraction of the wild type intestinal protein prep prior to anti-histone immunoprecipitation. Presumptive phospho-Mcm2 bands on this gel are denoted by the asterisk (*). (F) Western blot showing reduced Mcm2 in 96 hpf flo larvae, but normal levels in 96 hpf slj larvae compared with control wild type larvae. (G) Western blot showing inhibition of Chk2 phosphorylation in flo larvae treated with the Chk2 inhibitor. (H) Western blot showing specificity of the anti-phospho Chk2 antibody. Phospho-Chk2 levels are elevated in 84 hpf flo larvae but are reduced when injected with splice morpholinos (splice, two independent sets of injections shown) or morpholino designed against the Chk2 translation initiation site (atg) but not larvae injected with vehicle control (cntrl). (I) Western blot showing specificity of the anti-phospho Chk1 antibody: abundant phospho-Chk1 is present in irradiated Hela cells and the non-irradiated 96 hpf flo intestine, but reduced levels are present in the intestine of 96 hpf flo treated with the ATR inhibitor caffeine (10 μM; 15 μM beginning at 84 hpf); the intestine of 96 hpf flo larvae treated with caffeine (10 μM) and a commercially available ATM inhibitor (Sigma Aldrich; 12 μM); but not in the intestine of 96 hpf flo larvae treated with ATM inhibitor alone (12 uM beginning at 84 hpf). His3, anti-Histone 3; A/G, IG fraction recovered following protein A/G precipitation. |

Unillustrated author statements PHENOTYPE:
|