- Title
-
Atp7a determines a hierarchy of copper metabolism essential for notochord development
- Authors
- Mendelsohn, B.A., Yin, C., Johnson, S.L., Wilm, T.P., Solnica-Krezel, L., and Gitlin, J.D.
- Source
- Full text @ Cell Metab.
|
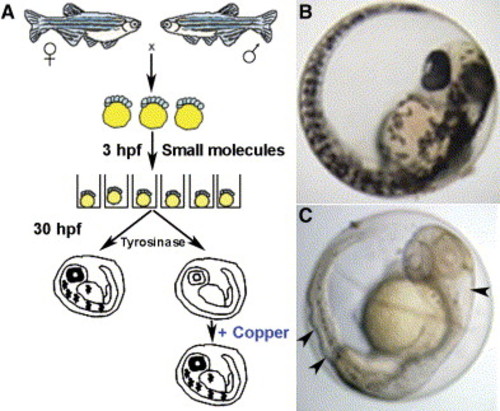
Schematic of the small-molecule screen for drugs perturbing copper metabolism A) Embryos from wild-type zebrafish were arrayed in a 96-well format and treated with drugs at 5 μM beginning at 3 hr postfertilization (hpf). At 30 hpf, the embryos were assayed for the development of melanin pigment. In a second screen, embryos were again exposed at 3 hpf to drugs that abrogated melanin formation but this time were supplemented with 25 μM CuCl2. In this screen, all drugs that prevented pigmentation in a manner reversible by the addition of exogenous copper were considered as perturbing copper homeostasis. B and C) As compared to wild-type embryos (B), copper-deficient embryos treated with neocuproine (C) lacked pigment and displayed a strikingly wavy notochord and enlarged hindbrain ventricle (arrowheads). |
|
Copper-deficient embryos display a pleiotropic phenotype DIC image of notochord from a control embryo at 28 hpf (A, left) reveals normal development of the central canal (c), floor plate (f), notochord (n), and aorta (a), while a copper-deficient embryo of the same age treated with neocuproine (NeoC) (A, right) shows malformations including distorted floor plate (arrow), undulating notochord, additional tissue structures (arrowhead), and displaced aorta. Alcian blue staining of 96 hpf control (B, left) and copper-deficient (B, right) embryos reveals reduced cartilage formation with compression (arrowhead) and impaired jaw elongation. At 48 hpf, fli1:GFP copper-deficient embryos (C) exhibit disorganization of the intersegmental vasculature with microaneurysms (arrowheads). O-dianisidine staining at 72 hpf (D) reveals a marked decrease in hematopoiesis (arrowheads) in a copper-deficient embryo (top) compared to control (bottom). DIC image at 32 hpf reveals an abnormal midbrain-hindbrain boundary (arrowheads) in copper-deficient (E, right) versus control (E, left) embryos. Antibody staining (Znp-1) reveals loss of primary motor neurons (arrow) and associated axons (arrowhead) in copper-deficient (F, right) versus control (F,left) embryo at 28 hpf. |
|
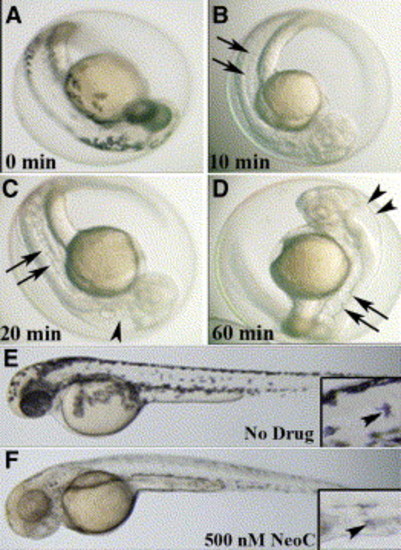
Small-molecule effects are rapid, saturable, and dose and time dependent Examination of embryo at 48 hpf following no treatment reveals normal melanin pigmentation and notochord (A), while treatment with 10 μM neocuproine for 10 min at 3 hpf abrogates melanin pigmentation but does not affect the notochord (arrows) (B). Treatment with 10 μM neocuproine for 20 min starting at 3 hpf reveals both abrogated pigmentation, enlarged hindbrain ventricle (arrowhead), and a wavy notochord (arrows) (C). Treatment with 10 μM neocuproine for 60 min at 3 hpf does not increase the severity of the pigment, notochord (arrows), or ventricle (arrowheads) phenotype (D), indicating that the effect is saturable. Untreated 48 hpf embryo (E) shows the normal distribution of melanocytes with melanin pigmentation (inset, arrowhead) and normal notochord, while treatment at 3 hpf with 500 nM neocuproine (one-twentieth of the dose of neocuproine resulting in the complete phenotype) (F) impairs only melanin pigmentation (inset, arrowhead). |
|
The zebrafish mutant calamityvu69 is defective in the zebrafish ortholog of the Menkes disease gene and is phenocopied by copper deficiency A and B) Copper-deficient embryo (A) and a cal homozygote (B) at 48 hpf. C and D) Phylogeny of copper-transport P type ATPases (C) displayed as maximum-likelihood tree with bootstrap values as shown. Aligned nucleotide sequence chromatograms (D) from genomic DNA from a portion of the atp7a gene from wild-type, cal heterozygote, and mutant embryos reveals a T→A transversion (box) in the intron 5′ to exon 9. E) Analysis of cDNA reverse transcribed from mRNA reveals that this mutation acts as a cryptic splice acceptor causing the insertion of seven nucleotides (TTCTCAG) into the mRNA. F) Restriction digests of a region amplified from atp7a cDNA reveal a novel DdeI site corresponding to the 7 bp insertion at the cal locus (arrowheads). EXPRESSION / LABELING:
|
|
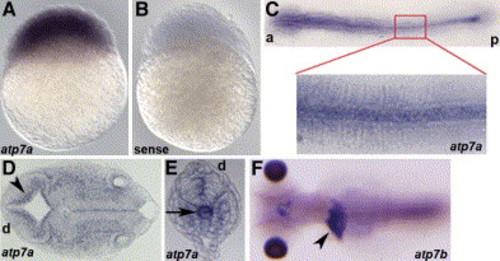
Expression pattern of atp7a via in situ hybridization Anterior (a), posterior (p), and dorsal (d) are as indicated. A) atp7a message is present in all cells at 3 hpf. B) Staining is specific, as a sense-control probe does not show staining. C) At 8 somites, atp7a is expressed most abundantly in the notochord and is expressed ubiquitously at lower levels. D and E) Sectioning at 24 hpf reveals staining along the ventricle (D) (arrowhead) and in the distal notochord (E) (arrow). F) In situ analysis of atp7b reveals expression in the liver (arrowhead) in a 5 dpf embryo as shown in this dorsal view. |
|
Rescue of cal with human ATP7A and transplantation experiments The calamity mutant phenotype (A) is rescued by injection with synthetic RNA encoding the human Menkes ATPase (B). Although cal embryos display a complete lack of melanin pigment at 48 hpf (C), transplantation of rhodamine-dextran-injected wild-type cells into cal embryos (D) yields embryos mosaic for pigmented melanophores (arrows). In all cases, the pigmented cells shown here in the retinal epithelium (E) correspond to the rhodamine-labeled donor cells as detected by fluorescence for CY3 in this same epithelium (F). |
|
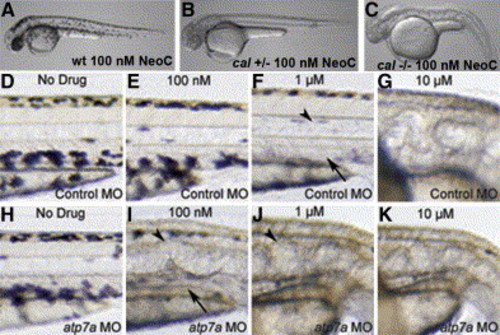
Interaction of atp7a dosage and developmental copper deficiency A and B) Wild-type embryos appear normal in 100 nM neocuproine (A); cal heterozygotes display loss of all melanin pigment at this same dose (B). C) The pleiotropic phenotype of copper deficiency is observed in all cal homozygotes in 100 nM neocuproine. D–K) Embryos injected with control MO (D–G) display a phenotypic dose-response curve in neocuproine of the notochord (arrowheads) and melanin pigment (arrows) that is altered in embryos injected with atp7a-targeted MO (H–K). All embryos were treated continuously from 3 hpf with the indicated dose of drug. |
|
Alignment of amino acid sequence of human and zebrafish atp7a Sequence alignment reveals 64.5% identity with marked conservation of CPC copper transport domain (underlined in black), all 6 MXCXXC copper-binding motifs (underlined in red), the invariant aspartate (underlined in blue) and the conserved SEHPL sequence required for trafficking (underlined in green). |
|
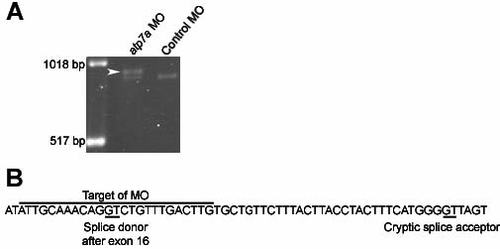
atp7a morpholino causes abnormal splicing of the zebrafish atp7a transcript A) PCR amplification from cDNA of the region surrounding the target of this MO reveals a single band of expected size from embryos injected with control MO, and an additional larger band (arrowhead) in embryos injected with MO to atp7a. B) Sequence analysis of this band reveals a transcript resulting from a cryptic splice donor 44 nt into the intron, causing a frame shift. The predicted resulting ORF, when translated, would contain 46 incorrect amino acids and terminate prematurely, removing the final 354 amino acids, including two transmembrane domains. EXPRESSION / LABELING:
|
Reprinted from Cell Metabolism, 4(2), Mendelsohn, B.A., Yin, C., Johnson, S.L., Wilm, T.P., Solnica-Krezel, L., and Gitlin, J.D., Atp7a determines a hierarchy of copper metabolism essential for notochord development, 155-162, Copyright (2006) with permission from Elsevier. Full text @ Cell Metab.