- Title
-
Aqp0a Regulates Suture Stability in the Zebrafish Lens
- Authors
- Vorontsova, I., Gehring, I., Hall, J.E., Schilling, T.F.
- Source
- Full text @ Invest. Ophthalmol. Vis. Sci.
|
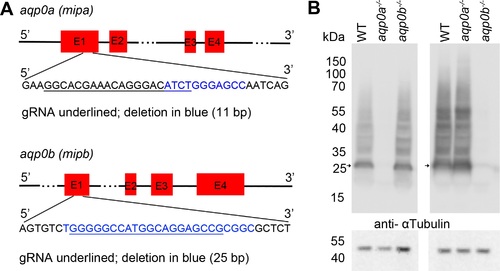
CRISPR-Cas9 mediated Aqp0a or Aqp0b functional knock-out. (A) gRNA designed against exon 1 of aqp0a or aqp0b co-injected with Cas9 resulted in out-of-frame deletions (11 bp for aqp0a and 25 bp for aqp0b) that are predicted to create premature stop codons. Mutants identified by PCR were confirmed by DNA sequencing. (B) Maternal-zygotic mutant adult lens fiber membrane protein was run on a Western blot. (B, top) Failure to detect at the monomeric molecular weight of ?28 kDa (arrow), as well as labeling of the polymeric bands was observed in aqp0a?/? by anti-Aqp0a antibody, and aqp0b?/? by anti-Aqp0b antibody, while the other ortholog was still detected. Stripping and reprobing the blot with anti-?-Tubulin antibody (B, bottom) served as a protein loading control. |
|
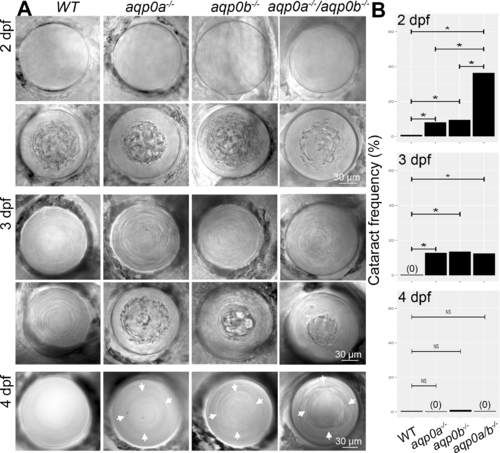
Loss of Aqp0a and/or Aqp0b increases frequency of embryonic cataract. (A) Representive in vivo DIC images of clear and cataractous lenses from WT, aqp0a?/?, aqp0b?/? or double mutant embryos taken at 2, 3, and 4 dpf. Refractive defects at the cortex-nucleus interface at 4 dpf are indicated by white arrows. (B) Cataract frequency was quantified at 2, 3, and 4 dpf by binary readout. A statistical test of proportions was used to estimate the difference in incidence rate of cataracts between each pair of genotype groups. The Holm method was used to calculate adjusted P values that account for multiple testing. Statistically significant differences at 95% confidence are indicated by an asterisk. See Supplementary Figure S1 for full statistical analyses.
|
|
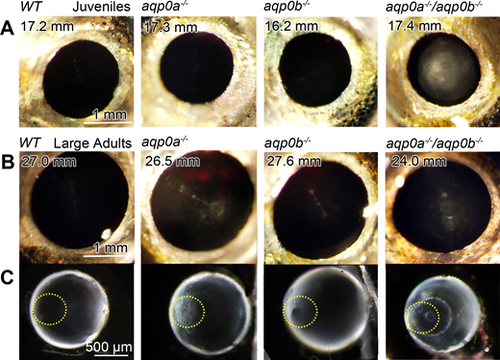
Cataracts form in double mutants and large aqp0a?/? mutants. Lens opacity assays were performed on live, anesthetized fish at a range of juvenile and adult stages. WT fish had no cataracts at any stage analyzed, even at very large adult stages. Double mutants had cataracts at all stages analyzed: (A) SL 8 to 11 mm (100%, n = 4 fish), 11 to 15 mm (100%, n = 6), 15 to 20 mm (100%, n = 11); (B) 20 to 23 mm (100%, n = 2), > 23 mm (100%, n = 3). At young stages, aqp0a?/? or aqp0b?/? mutants had no opacity ([A] shown at 15?20 mm SL), while large adult aqp0a?/? (> 23 mm) had consistent cataract (100%, n = 8) compared to much lower frequency in aqp0b?/? (25%, n = 8, lenses were typically clear as shown in [B]). Excised lenses from aqp0a?/? or double mutant large adults had anterior polar opacities (anterior pole is outlined with a yellow dotted line) while double mutants also had nuclear cataracts (C). Please see Supplementary Figure S2 for more examples of double mutant phenotypes.
PHENOTYPE:
|
|
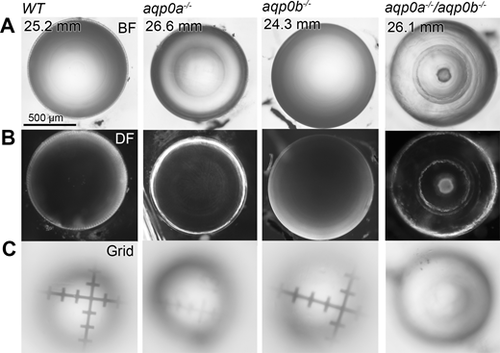
Aqp0a is required for normal lens optics. Representiative lenses dissected from WT, aqp0a?/?, aqp0b?/?, or double mutant zebrafish of SL >23 mm imaged through their optical axis (equatorial orientation). Lenses imaged under brightfield (BF; [A]) and darkfield (DF; [B]) illumination reveal a clear lens for WT and aqp0b?/? mutants, while aqp0a?/? mutants have mild refractive changes between the nucleus and lens periphery. Double mutant lenses have a severe nuclear cataract with cortical refractive problems. (C) Optical properties of lenses were tested by focusing through the lens onto micrometer grids, which revealed poor optical properties of the aqp0a?/? and double mutant lenses.
PHENOTYPE:
|
|
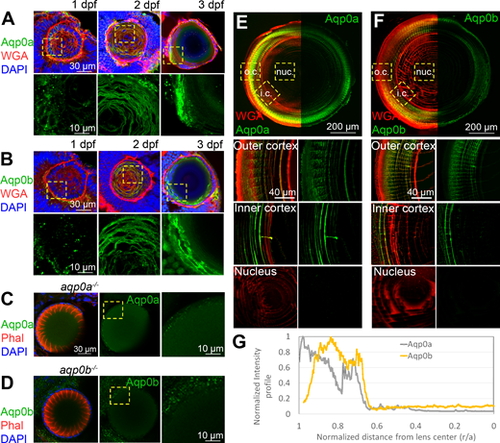
Expression of Aqp0a and Aqp0b in WT zebrafish lenses; 1 to 3 dpf eye cryosections labeled with (A) anti-Aqp0a or (B) anti-Aqp0b antibodies (green), plasma membrane label wheat germ agglutinin (WGA, red), and DAPI (blue). High power images of Aqp0a or Aqp0b labeling only were taken from regions outlined by yellow-dashed boxes. aqp0a?/? lenses labeled for F-actin (phalloidin, red) and DAPI (blue) as well as with (C) anti-Aqp0a antibody, and aqp0b?/? labeled with (D) anti-Aqp0b antibody serve as negative controls. Adult lens cryosections were labeled with (E) anti-Aqp0a or (F) anti-Aqp0b antibodies (green), and WGA (red). Equatorial lens section is shown with antibody labeling only detected in the cortex. High power images from the outer cortex (o.c.), inner cortex (i.c.), and nucleus (nuc.) are taken from regions outlined by yellow dashed boxes. (G) Normalized intensity of either Aqp0a (gray) or Aqp0b (yellow) antibody labeling in adult lenses as a function of distance from lens center. Each experiment is representative of at least six independent lenses analyzed.
PHENOTYPE:
|
|
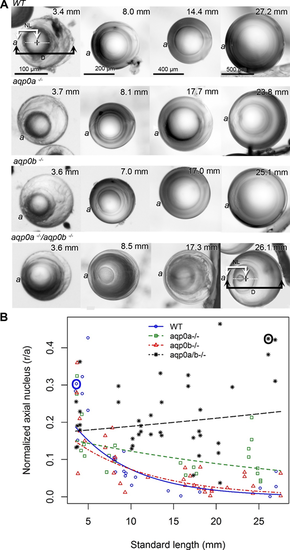
Aqp0a is required for lens nucleus centralization. (A) Lens sutures were used to orient lenses perpendicular to the optical axis (axial orientation). The anterior lens pole (a) is indicated (please note that although fish have laterally facing eyes, in this manuscript we are using ?anterior? as the part of the lens that sees light first). Representative lenses are shown from fish of indicated SL. Measurements were taken as shown in WT at 3.4 mm SL and double mutants at 26.1 mm SL, and resulting data points circled (in blue for WT, and black for double mutants in [B]). The distance from the center of the nucleus (plus) to the anterior pole (NL) was expressed as a ratio of lens radius (D/2). (B) The normalized distance of the center of lens nucleus to the anterior pole (r/a), where 1.0 is the lens surface, and 0.0 is the center of the lens, is graphed as a function of SL. Linear regression fits were used to assess the relationship between log normalized axial nucleus localization and SL. The estimated regression slope of aqp0a?/? was significantly different from WT (P = 9.85e-05) and aqp0b?/? (P = 0.00388). The estimated regression slope of double mutants was significantly different from WT (P = 7.55e-08) and aqp0b?/? (P = 5.21e-06). See Supplementary Figure S5 for full statistical analyses.
PHENOTYPE:
|
|
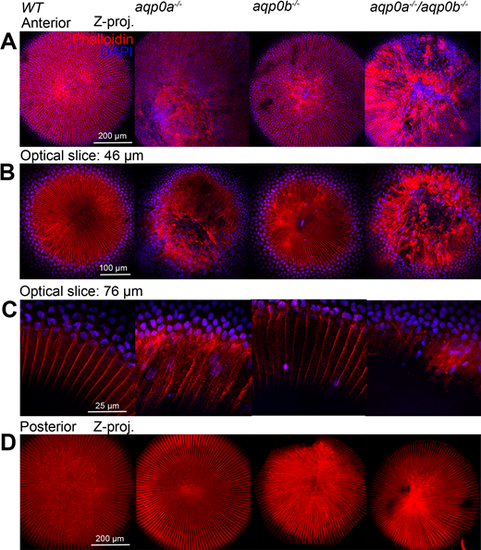
Aqp0a is required for anterior suture stability. Fixed and permeabilized large adult zebrafish lenses were labeled for F-Actin with phalloidin (red) and nuclei with DAPI (blue). Maximum z-projections were generated from 150 ?m z-stacks of the anterior pole (A) and 150 ?m z-stacks of the posterior pole (D) revealing the anterior, and posterior sutures, respectively. Optical slices 46 ?m from the anterior pole reveal detail of the anterior sutures, which are severely damaged in aqp0a?/? and double mutant lenses (B). High power images of peripheral fiber cells and epithelial cells from an optical slice taken 76 ?m from the anterior pole reveal the presence of nuclei in fiber cells in mutant lenses compared to WT (C). The cell nuclei within fiber cells counted in the whole optical slice taken 76 ?m for WT: 0, aqp0a?/?: 38, aqp0b?/?: 2, double mutants: 21.
PHENOTYPE:
|
|
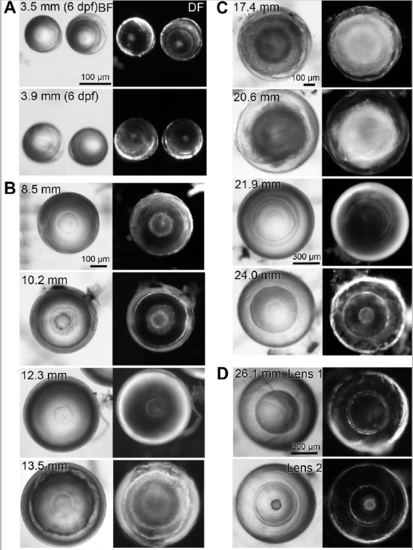
Variability in aqp0a/b double mutant lens phenotypes. Dissected double mutant lenses from zebrafish of specified standard length (SL) imaged through their optical axis (equatorial orientation) under brightfield (BF) and darkfield (DF) illumination. (A) The smallest dissectible lenses, at 6 dpf, already reveal nuclear cataracts. Lenses of a range of juvenile (B) and adult (C) SL reveal opacity of variable penetrance but always show nuclear cataract. (D) An example of a pair of lenses from a single fish. |
|
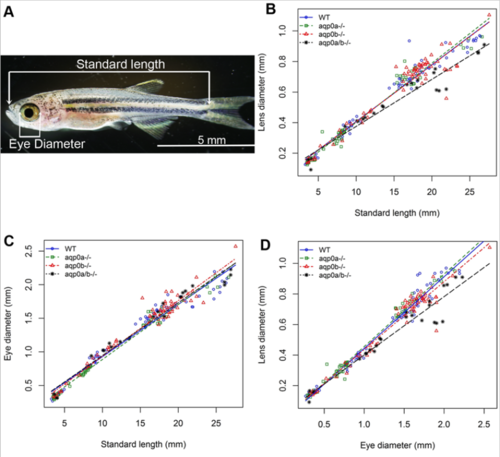
Slower growth of aqp0a/b double mutant zebrafish lenses. (A) Zebrafish standard length (snout to start of tail) was used to determine developmental stage in juvenile-adult fish. (B) Post-embryonic double mutant lenses grow more slowly than WT or single aqp0 mutants (p-values for estimated difference in estimated regression slopes: WT vs double mutant 0.000769; double mutant vs aqp0a-/- 6.87e-05; double mutant vs aqp0b-/- 0.00114; see Supp. Figure 1). (C) Eye growth rate was unaffected in the mutants, thus the lens diameter versus eye diameter also revealed a slower lens growth compared to eye diameter fo the double mutants. |