- Title
-
Zebrafish pronephros tubulogenesis and epithelial identity maintenance are reliant on the polarity proteins Prkc iota and zeta
- Authors
- Gerlach, G.F., Wingert, R.A.
- Source
- Full text @ Dev. Biol.
|
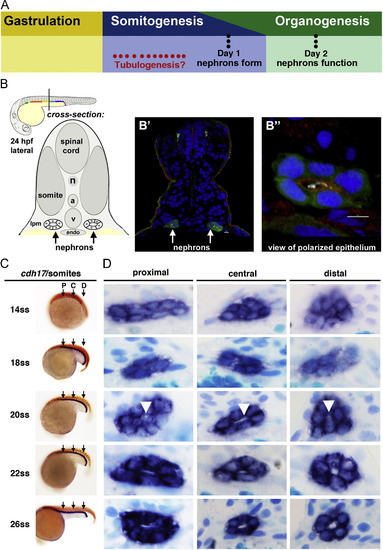
Renal progenitors exhibit a progression of rearrangements during nephrogenesis, and undergo tubulogenesis with lumen formation between the 20 and 22 ss time points. (A) Although the timing of tubulogenesis during pronephros development has been unresolved, we hypothesized that it transpires sometime during somitogenesis, as prior studies have demonstrated that nephron patterning is completed by embryonic day 1, and that pronephros function initiates at approximately day 2. (B) The zebrafish embryonic kidney is comprised of two bilateral nephrons that have a lumen at 24 hpf. (B′) Cross section of a zebrafish embryo in which the nephron tubule epithelial cells were labeled by IF to detect GFP (green) in the transgenic strain Tg(cdh17:eGFP), along with acetylated α-tubulin (light blue), PrkCI/? (red), and nuclei marked with DAPI (dark blue). (B") Digital zoom of a single nephron tubule, white bar indicates 5 um. (A-B′′) Schematics and images adapted with author rights (Gerlach and Wingert, 2013). (C) Renal progenitors were labeled in the developing pronephros between the 14 and 26 ss by performing WISH on wild-type embryos for the renal marker cdh17 (purple) and a somite marker (red) to confirm embryo stage (the somite marker myod1 was used embryos <18 ss and smyhc1 for embryos >18 ss). Black arrows indicate approximate region where proximal (P), central (C), or distal (D) cross-sections were analyzed. (D) Serial sections of WISH labeled embryos were collected and analyzed at proximal, central, and distal regions of the renal progenitor field. At 14 ss, renal progenitors were found in clusters of approximately ~8 cells. Between 18 and 22 ss, renal progenitors were found in circumferential clusters of ~6 cells. At the 20 ss, a small lumen was discernible (white arrowheads), and a clear lumen was subsequently visible in all regions of the pronephros at 22 ss that enlarged by 26 ss. EXPRESSION / LABELING:
|
|
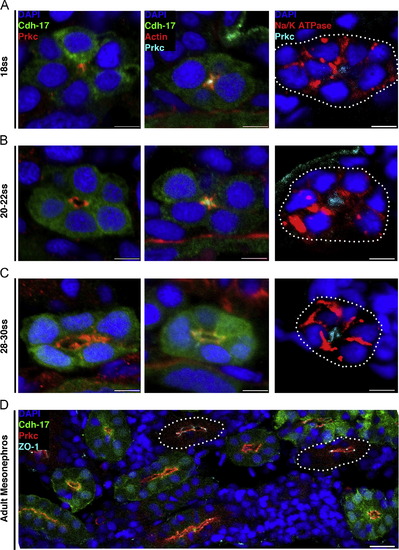
Changes in protein localization precede lumen formation in the pronephros, and similar polarity features characterize embryonic and adult zebrafish nephron tubules. Localization of proteins during nephrogenesis in wild-type transgenic Tg(cdh17:eGFP) zebrafish embryos and in the uninjured adult kidney. Tubules not labeled with GFP (due to incompatibility of particular antibody-fixative combinations) are outlined with white dots. (A) At the 18 ss: PrkCI/? and actin localized to the apical membrane domain prior to the appearance of a lumen, while Na+/K+ ATPase surrounded the cell membrane and was not excluded from the apical surface. (B) When a lumen formed at the 20?22 ss: PrkCI/? encompassed the apical membrane domain abutting the lumen, actin was found in a punctate pattern at the apical surface presumably localizing to junctional complexes, and Na+/K+ ATPase still surrounded a majority of the cell membrane but was not present in the region where PrkCI/? was localized. (C) At the 28?30 ss: PrkCI/? demarcated the apical membrane, actin was more band-like at the apical surface yet still exhibited punctate expression at cell junctions, and Na+/K+ ATPase showed a distinct basolateral membrane localization. (D) In the adult kidney, or mesonephros, PrkCI/? was located at the apical surface of tubule cells, and the tight junction marker ZO-1 showed strong labeling at junctional complexes. (A?C) Scale bars, 5 µm. (D) Scale bar, 20 µm. EXPRESSION / LABELING:
|
|
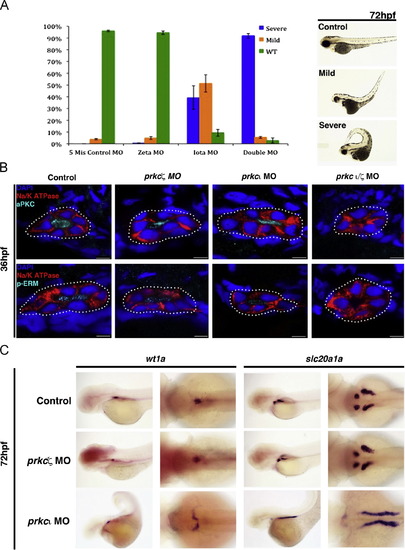
prkCI and prkc? are partially redundant during tubule polarity establishment, while prkCI is required for normal glomerulus development. (A) MO injections were performed with wild-type embryos to generate single prkCI, prkc?, and double prkCI/? knockdowns, and morphants were scored at 72 hpf for gross morphology compared to embryos injected with control double prkCI/? mismatch MOs. (Left) Percentage distributions of three classes of phenotypes, categorized as wild-type, mild, and severe. These categories were based on curled tail, edema, and patchy eye pigmentation (Right, lateral views of live representative embryos). (B) Confocal imaging of IF analysis during tubulogenesis in wild-type, prkCI, prkc? and prkCI/? morphant nephrons at the 36 hpf stage. Single morphants showed expression and proper localization of prkCI/? and p-ERM to the apical surface, whereas prkCI/? and p-ERM were absent in the double prkCI/? morphants. Na+/K+ ATPase was basolateral in wild-type embryos and single prkc morphants, but diffuse in the double prkCI/? morphants. Scale bars, 5 µm. (C) WISH analysis of podocyte and PCT development in wild-type control embryos and single prkc morphants using wt1a and slc20a1a, respectively. prkc? morphants had midline clusters of podocytes, similar to wild-type embryos, while prkCI morphants had scattered clusters of podocytes that failed to migrate to the midline. PCT coiling morphogenesis was normal in prkc? morphants and wild-type control embryos, while prkCI morphants had disrupted PCT coiling morphogenesis. (Left columns, lateral view; right columns, dorsal view.) EXPRESSION / LABELING:
PHENOTYPE:
|
|
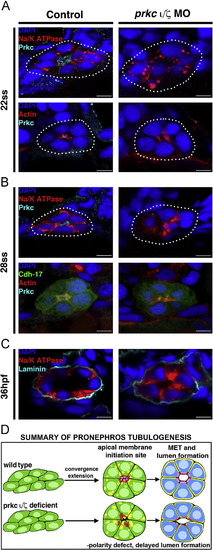
prkCI/? loss of function disrupts apical-basal protein localization and prevents proper polarity establishment, though delayed tubulogenesis eventually occurs. Protein localization during pronephros development between the 22 ss through 36 hpf stages revealed alterations in double prkCI/? morphant nephrons compared to wild-type embryo controls. (A) At the 22 ss, double prkCI/? morphants had disorganized actin and lack Prkc at the apical domain, while Na+/K+ ATPase was diffusely spread over the entire cell membrane in both wild-types and double morphants. (B) At 28 ss, the Na+/K+ ATPase was still diffusely spread over the cell membrane in the morphants, but in wild-types this protein was restricted to the basolateral cell surface in a mutually exclusive domain to that of prkCI/? and actin, which defined the apical domain. Double prkCI/? morphants continued to show disorganized actin and lack prkCI/? at the 28 ss. (C) At 36 hpf, wild-type control and double prkCI/? morphant tubules were surrounded by the ECM component laminin. prkCI/? morphant embryos had unrestricted Na+/K+ ATPase and a single lumen was present, albeit less pronounced than in wild-type controls. (A?C) Scale bars, 5 µm. (D) Summary of pronephros tubulogenesis. Renal progenitors emerge as groups of mesenchymal cells that undergo rearrangements into circumferential clusters that display differential distribution of apical membrane components prior to lumen formation, at which time mutually exclusive domains of apical and basolateral proteins are established. Deficiency of prkCI/? disrupts timely apical protein localization, delays lumen formation, and discrete apical and basolateral domains are not created. EXPRESSION / LABELING:
PHENOTYPE:
|
|
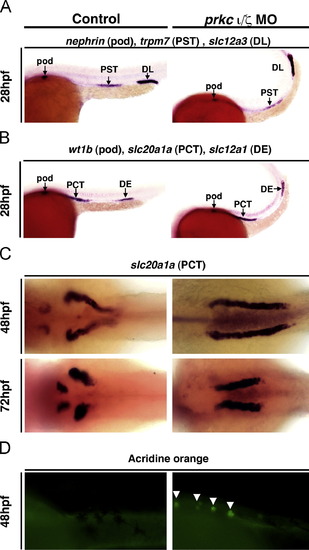
prkCI/? knockdown does not disrupt proximodistal segmentation, but does lead to defects in PCT morphogenesis. WISH was used to assess epithelial specification and segment pattern using antisense riboprobes (purple) in wild-types (left column) and prkCI/? morphants (right column). (A,B) In control and prkCI/? morphants the proximo-distal specification of nephron segments was normal. (Lateral views, anterior to the left) (A) Podocytes were labeled with nephrin, the proximal straight tubule (PST) with trpm7, and distal late tubule (DL) with slc12a3. (B) Podocytes were labeled with wt1b, the proximal convoluted tubule (PCT) with slc20a1a, and the distal early tubule (DE) with slc12a1. (C) In prkCI/? morphants the PCT failed to undergo proper morphogenesis and instead remained linear, while the PCT segment in control wild-type embryos at 48 hpf and 72 hpf underwent progressive coiling morphogenesis. (Dorsal views) (D) Acridine orange labeling indicates cell death in the PCT segment of the pronephros (white arrows) in prkCI/? morphants, but not wild-types. (Lateral views, anterior to the left.) EXPRESSION / LABELING:
PHENOTYPE:
|
|
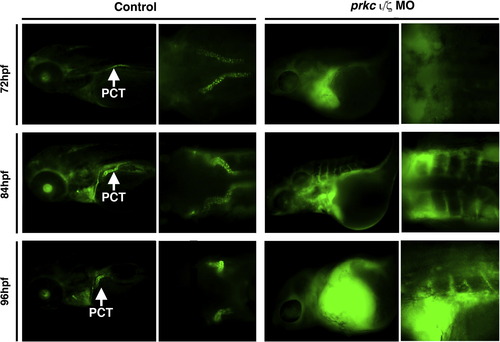
Renal clearance and PCT endocytosis are abrogated in prkCI/? deficient embryos. Embryos were injected with 40-kDa dextran?FITC at 48 hpf, and imaged at 72, 84, and 96 hpf. Control wild-type embryos displayed renal clearance by diminution of the net fluorescent signal intensity over time, as well as dextran?FITC internalization throughout the PCT (white arrows) during its progressive morphogenesis. DoubleprkCI/? morphants displayed severe fluid accumulation, notably pericardial edema that remained strongly positive for the dextran?FITC conjugate label, and the PCT was not labeled by dextran endocytosis (left panels, lateral views; right panels, dorsal views, with exception of bottom right which shows a lateral/dorsal angled view). PHENOTYPE:
|
|
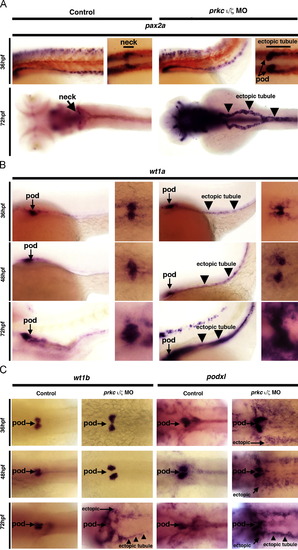
prkCI/? knockdown disrupts glomerular development and causes ectopic renal progenitor transcription factor expression. WISH was used to detect gene expression (purple) in wild-types (left column) and prkCI/? morphants (right column). (A,B) Left panels, lateral views; right panels, dorsal views; all embryos are shown with anterior to the left. (A) pax2a was restricted to the neck segment in wild-types, but showed ectopic podocyte and tubular expression (black arrowheads) in prkCI/? morphants at 36 and 72 hpf. (B) wt1a transcripts marked wild-type podocyte cells, but in prkCI/? morphants wt1a transcripts were also found in tubular cells (black arrowheads) and surrounding mesoderm cells. (C) wt1b and podxl were ectopically expressed in in prkCI/? morphants, though podxl showed ectopic expression beginning at 48 hpf and wt1b showed ectopic transcripts beginning at 72 hpf. Diffuse ectopic transcript expression (arrows) and ectopic tubule expression (black arrowheads) (dorsal views, anterior to the left).Renal clearance and PCT endocytosis are abrogated in prkCI/? deficient embryos. Embryos were injected with 40-kDa dextran?FITC at 48 hpf, and imaged at 72, 84, and 96 hpf. Control wild-type embryos displayed renal clearance by diminution of the net fluorescent signal intensity over time, as well as dextran?FITC internalization throughout the PCT (white arrows) during its progressive morphogenesis. Double prkCI/? morphants displayed severe fluid accumulation, notably pericardial edema that remained strongly positive for the dextran?FITC conjugate label, and the PCT was not labeled by dextran endocytosis (left panels, lateral views; right panels, dorsal views, with exception of bottom right which shows a lateral/dorsal angled view). EXPRESSION / LABELING:
PHENOTYPE:
|
|
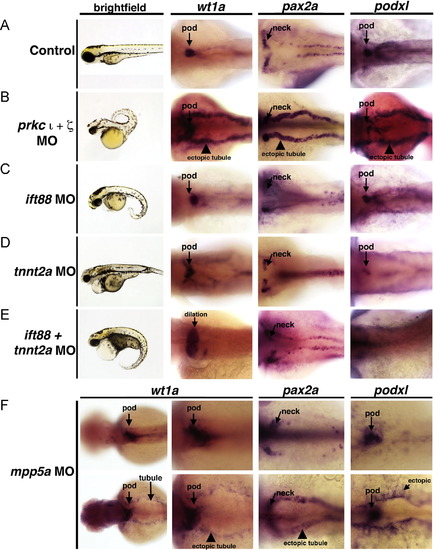
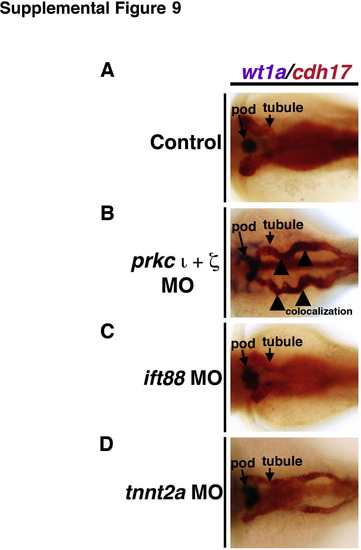
Ectopic expression of podocyte genes and early developmental transcription factors in the pronephros is not a general consequence of embryonic defects in fluid flow. WISH was used to assess gene expression using antisense riboprobes to detect wt1a, pax2a, or podxl (purple) in (A) wild-types, (B) prkCI/? morphants, (C) ift88 morphants, (D) tnnt2a morphants, (E) double ift88/tnnt2a morphants, and (F) mpp5a morphants. (A?E) Knockdown of prkCI/? was the only circumstance that led to high levels of ectopic expression of podocyte genes in the pronephros tubule. (F) (Top row) Most mpp5a morphants displayed no ectopic pronephros expression, but (bottom row) a subset (<10%) displayed low levels of ectopic wt1a, pax2a, or podxl in the tubule and also diffuse ectopic expression. EXPRESSION / LABELING:
PHENOTYPE:
|
|
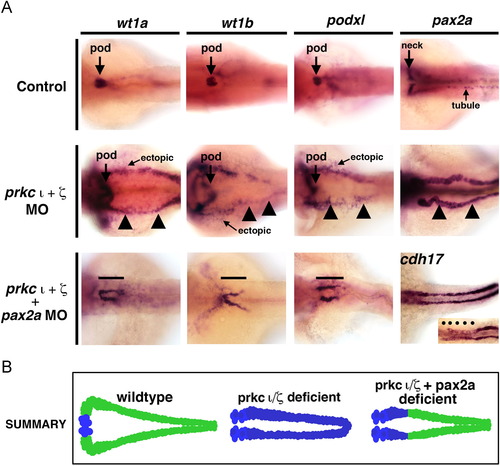
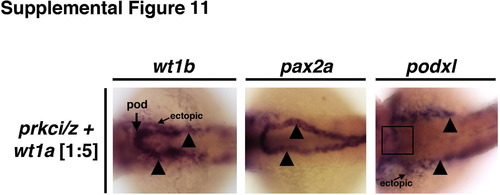
Ectopic expression of early developmental transcription factors in prkCI/? morphants is abrogated by concomitant knockdown of pax2a. WISH was used to assess gene expression using antisense riboprobes to detect wt1a, wt1b, podxl, pax2a or cdh17 (purple), in (A) wild-types, (B) prkCI/? morphants, or (C) prkCI/? morphants with combined pax2a knockdown. Wild-types showed restricted podocyte expression of wt1a, wt1b, and podxl, while pax2a was localized to the neck and intermittent pronephros tubule cells. prkCI/? morphants had high ectopic expression of each gene in the pronephros tubule. Knockdown of pax2a in prkCI/? morphants was associated with a short proximal pronephros domain of ectopic wt1a, wt1b, and podxl expression and low cdh17 transcript levels, while the tubule segment past this short ectopic segment domain was present and marked by a robust level of cdh17 transcripts. (D) Summary of ectopic misexpression during prkCI/? loss of function: wild-type embryos have mutually exclusive domains of podocyte versus tubule gene expression, prkCI/? morphants have ectopic misexpression of transcription factors throughout the pronephros, and pax2a knockdown represses this ectopic tubule misexpression in prkCI/? morphants, except for the neck region of the pronephros. EXPRESSION / LABELING:
PHENOTYPE:
|
|
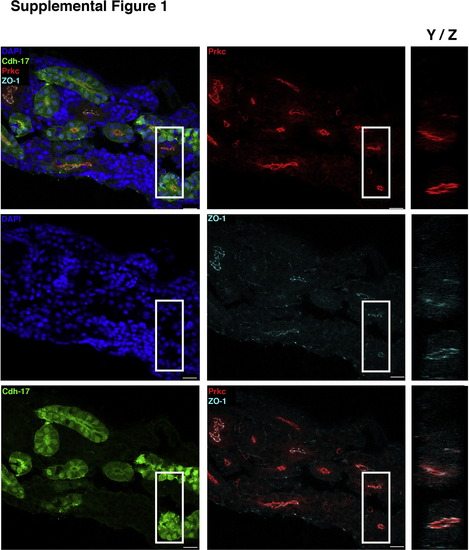
Localization of PrkCI/? in the mesonephric kidney. IF localization of proteins in the wild-type transgenic Tg(cdh17:eGFP) zebrafish adult kidney, a structure composed of nephrons and an interstitial stroma of hematopoietic precursors. (Top left panel) Composite overlay provided in Fig. 2D, with remaining panels showing individual channels or selected overlays. (Middle left panel) DAPI marks nuclei, and (bottom left panel) GFP marks most tubules, but is weak or absent in a subset of tubules due to transgene variegation. (Top middle panel) PrkCI/? was consistently found at the apical surface of tubule cells, and (middle panel) the tight junction marker ZO-1 showed strong labeling at junctional complexes. (Bottom middle panel) Overlay of PrkCI/? and ZO-1. (Right column) Panels show the Y/Z plane of the approximate area enlarged (demarcated by the white box), demonstrating that PrkCI/? and ZO-1 colocalize at the apical surface of tubules. Scale bars, 20 µm. |
|
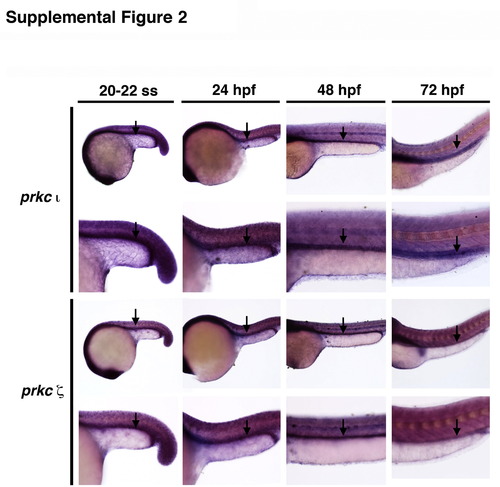
Expression of prkCI/? during zebrafish embryogenesis. Wild-type embryos displayed ubiquitous expression of transcripts encoding prkCI and prkC? at the 20 ss, 24 hpf, 48 hpf and 72 hpf stages of development. Black arrows demarcate the respective domains of prkCI and prkC? expression in the pronephros at each time point, which was somewhat elevated compared to adjacent tissues, such as those comprising the trunk. Embryos are shown in lateral views, with anterior to the left. |
|
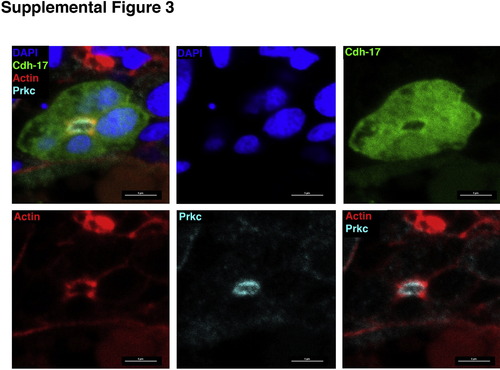
Co-localization of PrkCI/? and actin in the pronephros. Panels used to create composite image provided in Fig. 4B. (Top, left to right) Individual panels show composite overlay, DAPI and GFP. (Bottom, left to right) Individual panels show actin, Prkc, and the overlay of actin and Prkc. Scale bars, 5 µm. |
|
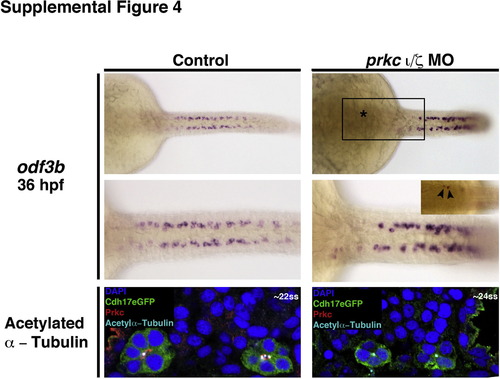
Expression of odf3b and acetylated α-tubulin in wild-type control and prkCI/? deficient embryos. WISH was used to evaluate the gene expression of odf3b (purple), a ciliogenesis marker, in wild-types (left top, middle panels) and double prkCI/? morphants (right top, middle panels) at the 36 hpf stage. Wild-type embryos and double prkCI/? knockdowns formed similar numbers and arrangements of odf3b-expressing cells, though occasional ectopic odf3b+ cells were observed in the proximal region of the pronephros in prkCI/? morphants (boxed area, top right panel, which is enlarged in the middle right panel). Asterisk indicates region where ectopic odf3b+ cells were located. (Bottom left, right panels) IF performed on cross sections of wild-types and double prkCI/? morphants at the 22?24 ss revealed that acetylated α-tubulin (light blue) was similarly present at the apical membrane of pronephros cells (labeled by GFP (green), nuclei marked with DAPI (dark blue), although double prkCI/? morphants lacked Prkc (red). |
|
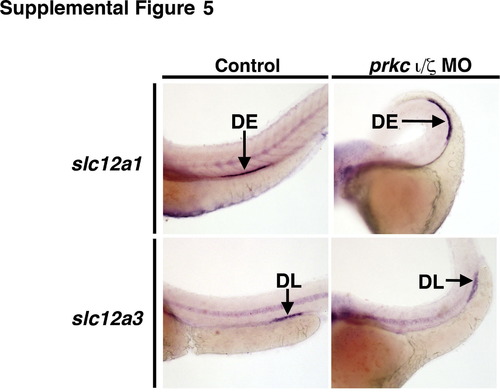
prkCI/? deficient embryos maintain distal segment domains at 72 hpf. WISH was used to evaluate the gene expression of the DE marker slc12a1 (purple) and the DL marker slc12a3 (purple), in wild-types (left panels) and double prkCI/? morphants (right panels). The domain of these segments were similar sizes in wild-types and prkCI/? knockdowns. |
|
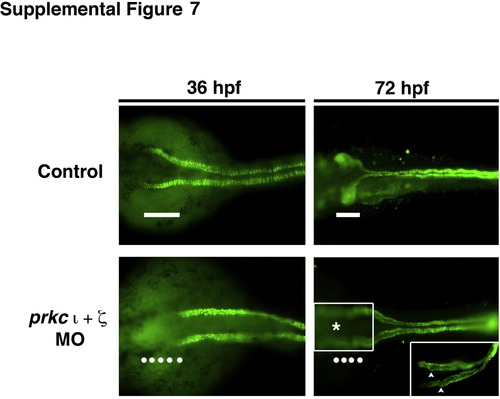
prkCI/? deficient embryos display reduced protein expression of the Na+/K+ ATPase in the neck region of the pronephros. Based on whole mount IF, wild-type embryos at 36 hpf exhibited Na+/K+ ATPase protein expression along the entire length of the pronephric tubule, including the proximal-most region (white bar). In contrast, double prkCI/? morphants at the 36 hpf stage fail to show Na+/K+ ATPase in the proximal most region of the pronephros (line of white dots). In wild-type embryos at 72 hpf, Na+/K+ ATPase continues to demarcate the entire tubule and shows the convoluted nature of the PCT (white bar), while prkCI/? deficient embryos have linear tubules that have failed to undergo normal morphogenesis. (Inset) Regions of the PCT in prkCI/? morphants exhibit folds and bulges (white arrowheads). |
|
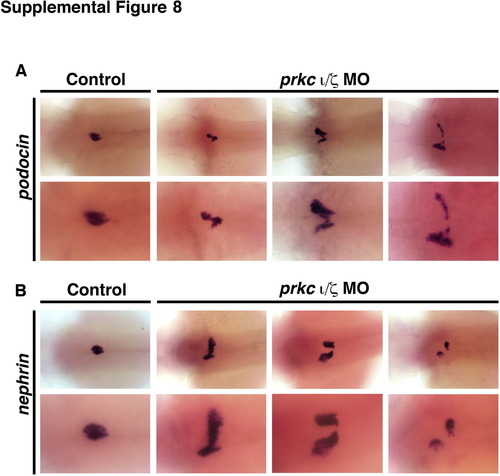
Expression analysis of podocyte specific transcripts nephrin and podocin show a range of failed migration and/or morphogenesis phenotypes in double prkCI/? morphant embryos. At 72 hpf, podocytes in wild-type controls (left panels) have migrated and fused at the midline to form a central glomerulus, that express (A) podocin and (B) nephrin (top row 4× magnification; bottom row 10× magnification). In double prkCI/? morphants, podocytes do not migrate normally to the midline, as shown by the pattern of (A) podocin and (B) nephrin expressing cells, and furthermore display variable migration patterns. |
|
Double WISH analysis of ectopic gene expression in the pronephros of fluid flow and cardiac contractility morphants. WISH was used to assess gene expression using antisense riboprobes to detect the combination of (A) wt1a (purple) and cdh17 (red) transcripts in (A) wild-types, (B) prkCI/? morphants, (C) ift88 morphants, and (D) tnnt2a morphants. Only prkCI/? morphants displayed overlapping expression of wt1a in cdh17-expressing nephron tubule cells. |
|
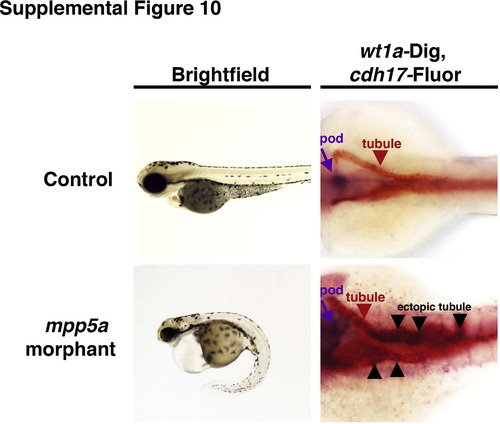
Analysis of mpp5a morphants and double WISH of ectopic gene expression in the pronephros. Brightfield images show live wild-type control and mpp5a morphant at 72 hpf (lateral view, anterior to the left). WISH panels show wt1a (purple) and cdh17 (red). Wild-type embryos have mutually exclusive domains of wt1a in podocytes and cdh17 in the tubule, respectively. The minor class of mpp5a morphants with ectopic pronephros gene expression of wt1a has these transcripts located in the cdh17+ tubule (black arrowheads denote areas of co-staining). |
|
Knockdown of wt1a in prkCI/? morphant embryos is not sufficient to ameliorate ectopic tubule expression. At 72 hpf, high levels of ectopic expression of transcripts encoding wt1b, pax2a and podxl are present throughout the tubule domain of prkCI/? morphant embryos. Knockdown of wt1a dramatically abrogates podxl transcript levels in the podocyte population (boxed area). |
|
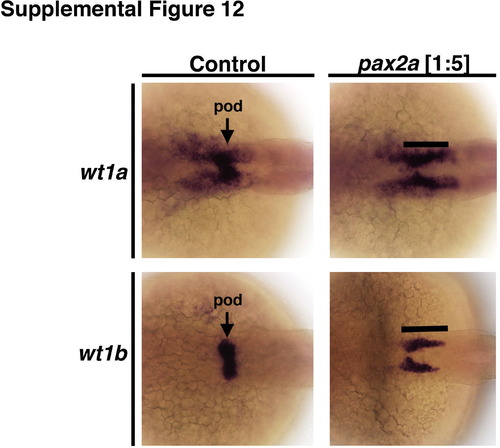
pax2a morpholino knockdown recapitulates the phenotypes displayed by noi homozygous mutant embryos, and was not associated with ectopic pronephros expression of podocyte genes in proximal and distal tubule domains. In 28 hpf wild-type embryos, wt1a and wt1b are expressed in the podocyte clusters with wt1a transcripts extending rostrally outside the podocyte domain. Conversely, pax2a morphant embryos have both wt1a and wt1b transcripts in an expanded caudal domain that phenocopies noi mutant embryos. |
|
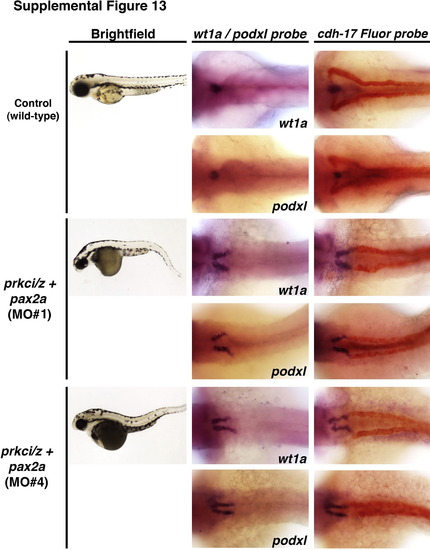
Two other independent pax2a morpholinos rescue ectopic gene expression in the pronephros of prkCI/? deficient embryos. (Left column) Brightfield images show live wild-type control and prkCI/?+pax2a knockdown embryos at the 72 hpf stage (lateral view, anterior to the left). (Middle column) WISH panels show wt1a or podxl (purple) in wild-type embryos and triple morphants. (Right column) Double WISH panels show wt1a or podxl (purple) and cdh17 (red) in wild-type embryos and triple morphants. Knockdown of pax2a, with either previously published MO1 or MO4, was associated with ectopic neck expression of wt1a or podxl that was exclusive of the cdh17+ tubule domain. |
Reprinted from Developmental Biology, 396(2), Gerlach, G.F., Wingert, R.A., Zebrafish pronephros tubulogenesis and epithelial identity maintenance are reliant on the polarity proteins Prkc iota and zeta, 183-200, Copyright (2014) with permission from Elsevier. Full text @ Dev. Biol.