- Title
-
Zebrafish foxe3: Roles in ocular lens morphogenesis through interaction with pitx3
- Authors
- Shi, X., Luo, Y., Howley, S., Dzialo, A., Foley, S., Hyde, D.R., and Vihtelic, T.S.
- Source
- Full text @ Mech. Dev.
|
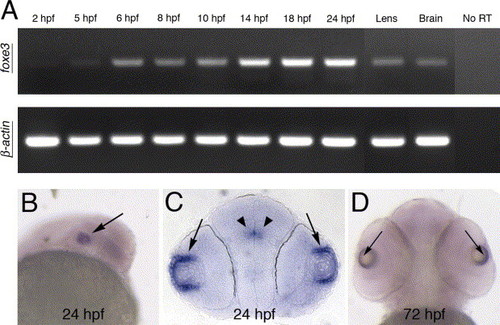
Temporal and spatial expression of the foxe3 gene. (A) RT-PCR demonstrates the temporal expression of foxe3 during embryonic development. A low level of maternal foxe3 transcript is detected (2 hpf) and foxe3 expression levels increase at 14?18 hpf. In addition, foxe3 transcription is detected in adult lens and brain. As a control, β-actin amplification is shown in the lower panel. (B?D) Whole-mount in situ hybridization demonstrates foxe3 spatial expression at 24 and 72 hpf. Expression of foxe3 is largely restricted to the eye (panel B, arrow) with highest transcript levels detected in the lens epithelial cells at 24 and 72 hpf (arrows in panels C and D, respectively). Restricted expression within the brain is also detected at 24 hpf (panel C, arrowheads). |
|
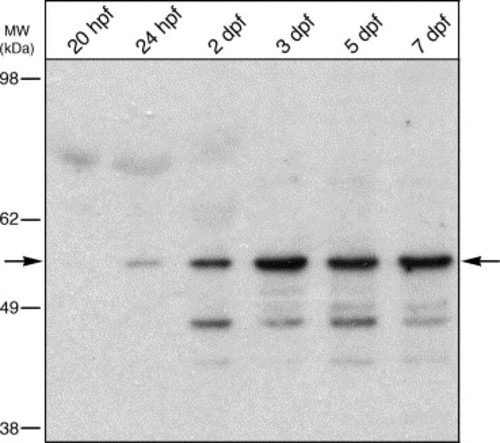
Temporal expression of Foxe3 protein. Immunoblot detection of Foxe3 in extracts from 20 and 24 hpf whole embryos and heads at 2, 3, 5 and 7 dpf. The Foxe3 protein is barely detected at 20 and 24 hpf (arrow). The expression level increases through 7 dpf compared to the earlier time points. Relative location of molecular weight markers are shown to the left (in kDa). |
|
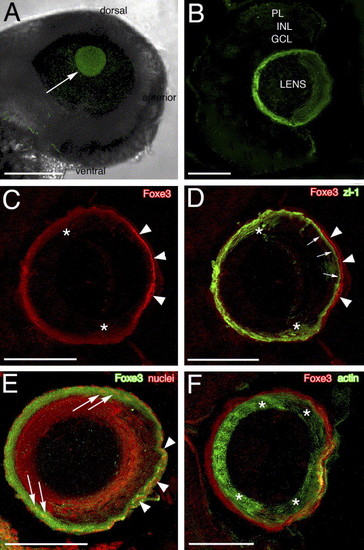
Foxe3 immunolocalization. The purified Foxe3 antiserum was used to localize protein expression in the lens by whole-mount labeling of wild-type embryos (panel A) and 4 and 5 dpf frozen larval eye sections (panels B?F). The Foxe3 protein is detected in the lens at 48 hpf (panel A, arrow). On frozen eye sections at 4 dpf, the majority of Foxe3 protein in the lens is localized to the most peripheral cells and the protein is not detected in the retina (panel B). The double immunolocalization of Foxe3 and zl-1 is shown in panels C and D. The Foxe3 signal (panel C, red) is most intense in the region of the distal (anterior) epithelial cells (arrowheads) with more diffuse labeling in the region corresponding to the cortical fibers and the elongating fiber cells (asterisks). In the overlay of the Foxe3 and zl-1 patterns (panel D), zl-1 expression (green) is detected in the elongating fiber cells (asterisks) and at the base of the distal epithelial cells (arrows). Foxe3 expression in the distal epithelial cells (arrowheads) does not overlap with the strong zl-1 signal in the cortical fibers at the base of these epithelial cells (arrows). In panel E, the anti-Foxe3 labeling (green) overlaps with propidium iodide-stained lens cell nuclei (red) in the region of the elongating fiber cells (arrows) and the epithelial cells (arrowheads). In comparison, Foxe3 expression (red) does not overlap with the majority of the actin localized in the differentiated fiber cells (green) of the lens cortex (panel F, asterisks). Scale bars = 125 μm (panel A) or 50 μm (panels B?F). Abbreviations: PL, photoreceptor layer; INL, inner nuclear layer and GCL, ganglion cell layer. |
|
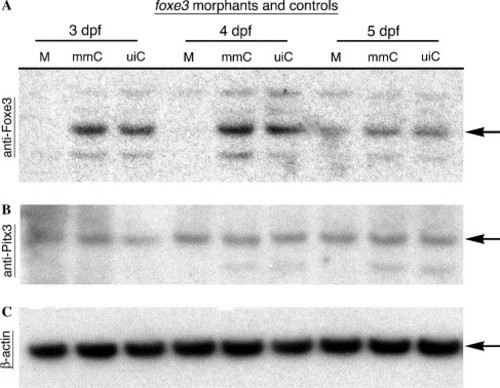
Immunoblot analysis of morpholino-mediated Foxe3 knockdown. Protein was extracted from foxe3 morphants and controls for immunodetection using anti-Foxe3, anti-Pitx3 or anti-β-actin serum. At 3 and 4 dpf, Foxe3 protein is not detected in the morphants (M), while wild-type Foxe3 levels are expressed in the mismatch control morpholino-injected (mmC) and uninjected (uiC) controls. At 5 dpf, nearly equivalent amounts of Foxe3 are detected in the foxe3 morphant and both controls. In comparison, the foxe3 morphants express wild-type levels of both Pitx3 and β-actin at all three ages. EXPRESSION / LABELING:
|
|
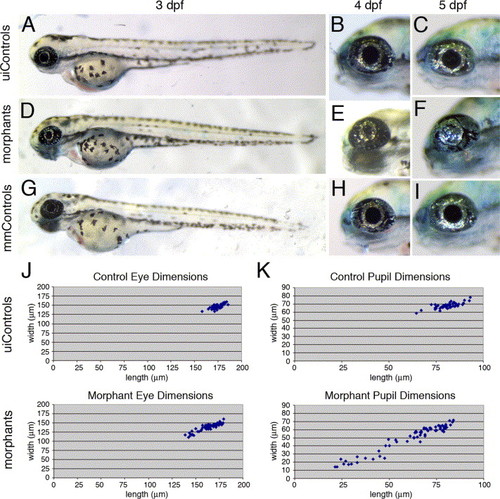
The foxe3 morphants display reduced eye and pupil size. The foxe3 morphants (panels D-F) are compared to uninjected (panels A?C) and mismatch control morpholino-injected embryos (panels G?I) at 3, 4 and 5 dpf. At 3 dpf, the eyes of the foxe3 morphants (panel D) are slightly reduced in size compared to the controls (panels A and G). At 4 dpf, small pupils also characterize the morphants (compare panel E to panels B and H). The eyes of some morphants lacked a pupillary opening at 5 dpf (compare panel F to panels C and I). The distribution of eye dimensions that were measured along the anterior-posterior axis (length) and dorsal?ventral axis (width) of uninjected control and foxe3 morphants (5 dpf) are shown in panel J. The distributions of control and foxe3 morphant pupil dimensions are also shown (panel K). PHENOTYPE:
|
|
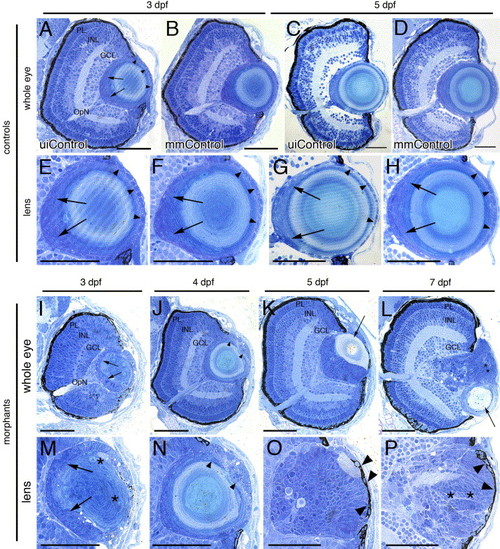
Histology of the foxe3 morphant eyes. Plastic resin-embedded sections of controls (panels A?H) and foxe3 morphants (panels I?P) were stained with methylene blue/azure II. At 3 and 5 dpf, the wild-type retina is laminated into three nuclear layers (panels A and C, uiControl). High magnification images (panels E and G) demonstrate the wild-type lens organization is characterized by a single layer of epithelial cell nuclei (arrowheads) surrounding the central lens fiber cells. Nuclei of elongating fibers are identified near the proximal lens pole (arrows). The retinal and lens morphologies of the mismatch control morpholino-injected (mmControl, panels B, D, F and H) embryos did not differ significantly from the wild-type eye morphology. Sections of the foxe3 morphant eyes at 3, 4, 5 and 7 dpf are shown in panels I?L with higher magnification views of morphant lenses shown in panels M-P. At 3 dpf (panels I and M), the morphant lens epithelial cell nuclei are enlarged, disorganized and multilayered (arrows). In addition, some fiber cells are swollen with retained nuclei (asterisks). These epithelial cell abnormalities are also evident at 4 dpf (panels J and N, arrowheads). At 5 dpf, the morphant lens exhibits a more homogenous appearing population of nucleated cells (panels K and O) with a relatively small fiber cell mass (panel K, arrow). At 7 dpf, the nucleated cells of the morphant lens appear organized into layers (panels L and P, asterisks). Some morphant lens epithelial cells also exhibit pigmentation at 5 and 7 dpf (panels O and P, arrowheads). The morphant lenses shown at high magnification in panels M, O and P are from different morphant eyes than those shown in panels I, K and L, while the lens in panel N is the same as shown at lower magnification in panel J. Scale bars = 50 μm. Abbreviations: PL, photoreceptor layer; INL, inner nuclear layer; GCL, ganglion cell layer; OpN, optic nerve; uiControl, uninjected control and mmControl, mismatch morpholino-injected control. PHENOTYPE:
|
|
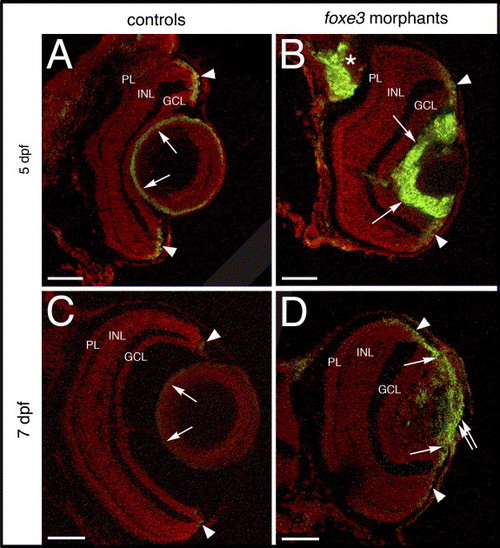
Cell proliferation in the foxe3 morphant lens. Frozen sections from control and foxe3 morpholino-injected embryos were immunolabeled for PCNA, a marker for proliferating cells. The control lens exhibits very few PCNA-positive cells at 5 and 7 dpf (panels A and C, respectively; arrows). Large numbers of anti-PCNA-labeled cells are identified in the foxe3 morphant lens at these ages (panels B and D, arrows). Small groups of PCNA-expressing cells are identified in the retinal circumferential marginal zones of both control and morphant eyes (panels A?D, arrowheads). A region of PCNA-labeled cells extends from the iris across the lens in the 7 dpf foxe3 morphant (panel D, double arrow). In addition, a region of cell proliferation in the brain is identified in the 5 dpf foxe3 morphant section (panel B, asterisk). Scale bars = 50 μm. Abbreviations: PL, photoreceptor layer; INL, inner nuclear layer and GCL, ganglion cell layer. |
|
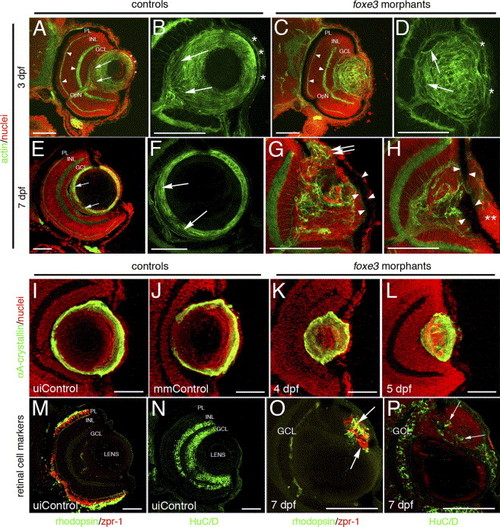
Immunohistochemical analysis of foxe3 morphant lens and retina. Frozen eye sections of uninjected controls (panels A, B, E and F) and foxe3 morphants (panels C, D, G and H) were stained with phalloidin-AF488 and propidium iodide to label actin filaments and cell nuclei, respectively. At 3 dpf in the uninjected control, actin is localized in the retinal plexiform layers (panel A, arrowheads) and the membranes of the lens cells (panel A, low magnification; panel B, high magnification). The phalloidin-stained 3 dpf control lens (panel B) demonstrates actin localization in the elongating lens fibers near the proximal lens pole (arrows) and epithelial cell membranes (asterisks). The 3 dpf morphant retinal structure is equivalent to the control based on the actin staining pattern (compare panel C to A, arrowheads), while the morphant lens actin localization pattern is abnormal (panels C and D). At 3 dpf, the morphant lens (panel D) exhibits a failure of lens fiber elongation (arrows) and disorder in the fiber cell and epithelial cell (asterisks) populations. At 7 dpf, the control lens actin staining (panels E and F) is restricted to the cortical fibers (green) and overlaps with the more peripheral population of nucleated cells (panel E, low magnification; panel F, high magnification shows only the actin staining). The region of elongating fiber cells can be identified (panels E and F, arrows). Two different 7 dpf morphant lens are shown in panels G and H, with both revealing many nucleated cells (red) that fail to express actin (green). Nucleated cells are contiguous between the morphant lens and the cornea (panel G, double arrows). In addition, pigmented cells cover the lens (panels G and H, arrowheads) and the corneal nuclear arrangement is abnormal (panel H, asterisks). αA-crystallin protein was immunolocalized in the lens of control and foxe3 morphants (panels I?L). In the uninjected and mismatch control morpholino-injected embryos (panels I and J, respectively) at 5 dpf, high levels of αA-crystallin protein (green signal) are detected in the lens periphery. αA-crystallin is also detected in the morphant lens at 4 and 5 dpf (panels K and L, respectively), although the localization pattern differs from the controls (compare panels K and L to I and J). Retinal cell antigens were also immunolocalized in control and foxe3 morphant frozen sections (panels M?P). In the controls, rhodopsin and zpr-1 antibodies label the rod and double cone photoreceptors, respectively (panel M, green and red signals, respectively), while HuC/D protein is detected in differentiated amacrine and ganglion cells (panel N, green signal). Some morphant lens display aberrant expression of retinal cell markers. The morphant lens in panel O exhibits rhodopsin and zpr-1 expression (arrows), while the morphant lens in panel P displays abberant HuC/D expression (arrows). The green signal on the posterior surface of the lens in panel O represents autofluorescence and not primary antibody detection. Scale bars = 50 μm. Abbreviations: PL, photoreceptor layer; INL, inner nuclear layer; GCL, ganglion cell layer; OpN, optic nerve; uiControl, uninjected control and mmControl, mismatch morpholino-injected control. EXPRESSION / LABELING:
PHENOTYPE:
|
|
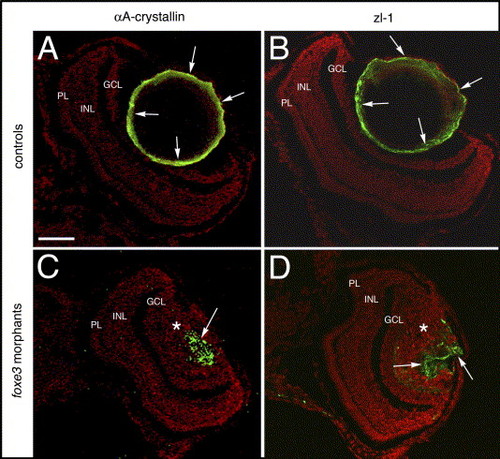
Lens cell marker expression in 7 dpf foxe3 morphants. Frozen sections from control (panels A and B) and morpholino-injected (panels C and D) embryos were immunolabeled for αA-crystallin and the zl-1 antigen at 7 dpf. In the controls, high levels of these proteins are detected in the peripheral lens cells (panels A and B, arrows). In the morphant lens, a subpopulation of cells express these proteins in an aberrant pattern (panels C and D, arrows). The asterisks in panels C and D mark the nucleated lens cell population. The scale bar (50 μm) in panel A applies to all panels. Abbreviations: PL, photoreceptor layer; INL, inner nuclear layer and GCL, ganglion cell layer. |
|
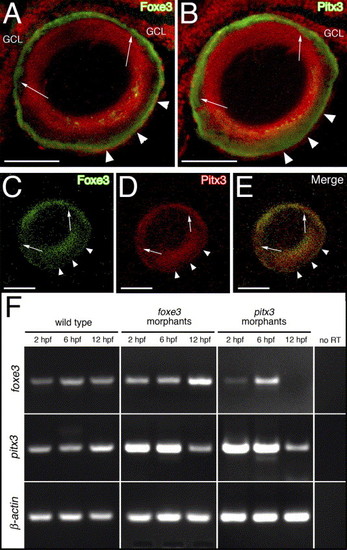
Relationship between Foxe3 and Pitx3. Foxe3 and Pitx3 protein immunolocalization in 5 dpf larval frozen sections is shown in panels (A?E). Immediately adjacent sections were labeled with either Foxe3 or Pitx3 polyclonal antiserum (panels A and B, respectively; green signal). The two different antibodies detect their respective proteins in nearly identical patterns on these sections (arrows and arrowheads; nuclei were stained with propidium iodide [red signal]). The purified Foxe3 and Pitx3 antibodies were conjugated to either AlexaFluor-488 or -594, respectively and used to colabel lens sections (panels C?E). The individual signals corresponding to Foxe3 (green, panel C) or Pitx3 (red, panel D) overlap in the merged image (panel E). Scale bars = 50 μm. Abbreviations: GCL, ganglion cell layer. RT-PCR was used to assess foxe3 and pitx3 gene expression in the foxe3 or pitx3 morphants at 2, 6 and 12 hpf (panel F). Foxe3 and pitx3 transcripts are detected at all three time points in wild-type embryos. In addition, both foxe3 and pitx3 transcripts are detected in the foxe3 morphants. In contrast, foxe3 transcripts are not detected in the pitx3 morphants at 12 hpf. Control RT-PCRs lacking reverse transcriptase (no RT) failed to amplify any of the three targets. |
|
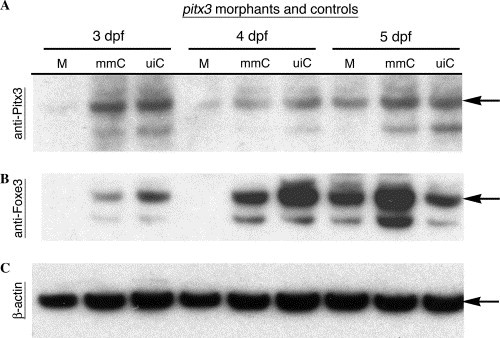
Foxe3 protein expression is reduced in pitx3 morphants. Immunoblot analysis of protein expression in the pitx3 morphants was used to further assess possible genetic interactions between pitx3 and foxe3. The pitx3 morphants (M) lacked Pitx3 protein at 3 and 4 dpf, while wild-type levels of Pitx3 were detected in both the mismatch control morpholino-injected (mmC) and uninjected (uiC) controls (panel A; arrow). At 5 dpf, Pitx3 protein was detected in the pitx3 morphants and the controls. Panel B demonstrates that Foxe3 protein is not detected in the pitx3 morphants (M) at 3 and 4 dpf, but is present in both controls (mmC and uiC). In 5 dpf morphants, Pitx3 and Foxe3 are present at nearly normal levels (panels A and B, respectively). Actin detection within the extracts demonstrates equivalent protein loading of the lanes (panel C). EXPRESSION / LABELING:
|
|
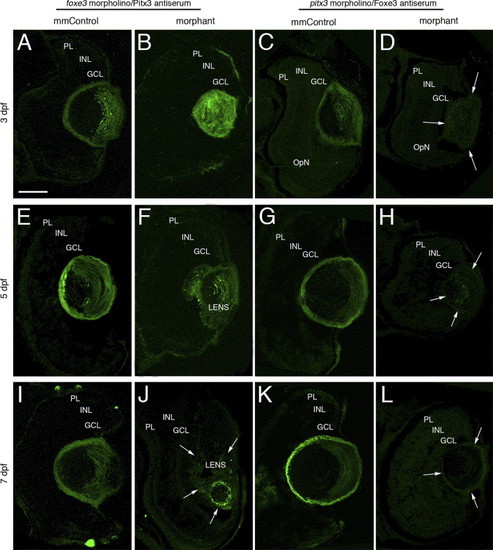
Immunolocalization of Pitx3 and Foxe3 in foxe3 and pitx3 morphant eye tissues. The Pitx3 and Foxe3 proteins were immunolocalized in frozen sections of either foxe3 or pitx3 morphant eyes and the relevant mismatch control morpholino-injected embryos (mmControl). Pitx3 is detected in the lens of foxe3 mmControl embryos at 3, 5 and 7 dpf (panels A, E and I, respectively), and also in the foxe3 morphant lens at these same time points (panels B, F and J). The Pitx3 spatial expression pattern is disrupted in the foxe3 morphants. A wild-type Foxe3 expression pattern is displayed in the pitx3 mmControl embryos at 3, 5 and 7 dpf (panels C, G and K, respectively). In contrast, Foxe3 protein is not detected in the pitx3 morphants at 3, 5 and 7 dpf (panels D, H and L, respectively; arrows delineate the lens tissue). The scale bar in panel A (50 μm) applies to all the panels. Abbreviations: PL, photoreceptor layer; INL, inner nuclear layer; GCL, ganglion cell layer. EXPRESSION / LABELING:
|
Reprinted from Mechanisms of Development, 123(10), Shi, X., Luo, Y., Howley, S., Dzialo, A., Foley, S., Hyde, D.R., and Vihtelic, T.S., Zebrafish foxe3: Roles in ocular lens morphogenesis through interaction with pitx3, 761-782, Copyright (2006) with permission from Elsevier. Full text @ Mech. Dev.