- Title
-
Sox4 regulates choroid fissure closure by limiting hedgehog signaling during ocular morphogenesis
- Authors
- Wen, W., Pillai-Kastoori, L., Wilson, S.G., Morris, A.C.
- Source
- Full text @ Dev. Biol.
|
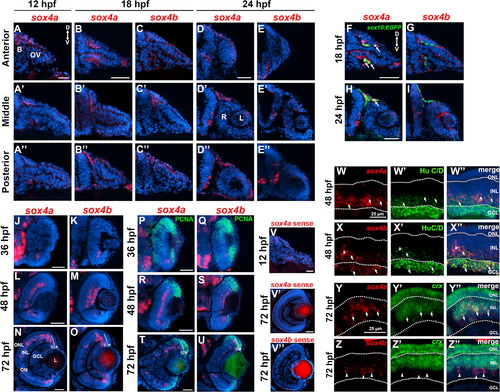
Expression of sox4a and sox4b during ocular development. (A?A′′) Fluorescent in situ hybridization (FISH) for sox4a performed on transverse cryosections at 12 hpf. (B?D′′) FISH for sox4a (B?B′′ and D?D′′) or sox4b (C?C′′ and E?E′′) performed on transverse cryosections taken at the level of the anterior, middle, and posterior optic vesicle at 18 or 24 hpf. (F?I) FISH with sox4a and sox4b probes performed on sections from sox10:EGFP transgenic embryos at 18 and 24 hpf. (J?O) FISH for sox4a or sox4b performed on transverse retinal cryosections at 36, 48, and 72 hpf. (P?U) FISH combined with immunohistochemistry for the proliferation marker PCNA at 24, 48, or 72 hpf. (V?V′′) Control FISH with a sense probe for sox4a and sox4b at 12 and 72 hpf. (W?X′′) FISH combined with immunohistochemistry for HuC/D, which labels ganglion and amacrine cells, at 48 hpf. (Y?Z′′) Two-color FISH with sox4a/b and crx probes performed at 72 hpf. Arrows indicate co-localization, arrowheads indicate sox4a/b+ cells that did not co-localize with the indicated marker. All scale bars in A-V equal 50 µm. D, dorsal; V, ventral; B, brain; OV, optic vesicle; L, lens; R, retina; P, peripheral CMZ; C, central CMZ; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; ON, optic nerve. EXPRESSION / LABELING:
|
|
Knockdown of sox4 causes coloboma. Representative images of control MO-injected embryos (A?D), sox4 MO-injected embryos (A2?D2), and embryos injected with both sox4 MO and sox4 mRNA (A′′?D′′). A minimum of 5 embryos were imaged for each group. Images in A?C′′ are lateral views; images in D?D′′ were taken from the ventral side of the embryo. Insets show the whole body of the same embryo in the larger panel. Arrow in A′ indicates the horizontal crease; arrows and brackets in B?C′′ indicate the choroid fissure. (E-F) Transverse DAPI-stained sections of control and sox4 morphants at 72 hpf. The coloboma in the sox4 morphant retina is prominent (F, asterisk). (G) Quantification of the coloboma phenotype at 48 hpf. In control morphants, 0.61±0.49% of embryos displayed coloboma (n=2/329). In sox4 morphants, 45.01±4.43% of embryos displayed coloboma (n=257/571). Co-injection of sox4 mRNA significantly reduced the incidence of coloboma to 16.49±3.37% (n=31/188; Fisherós exact test, P<0.0001). Scale bars for the insets in B?C′′ equal 500 µm. PHENOTYPE:
|
|
Persistence of laminin expression at the choroid fissure and altered proximo-distal patterning of the optic vesicle in sox4 morphants. (A?D′) Laminin immunostaining on control and sox4 morphant embryos at 48 and 72 hpf. Representative images from 15?20 individuals analyzed for each group are shown. (E?H) Double FISH for pax2a and pax6a at 18 and 48 hpf. (I) qPCR analysis revealed a 1.69-fold increase in pax2a expression and a 2.0-fold decrease in pax6a expression in sox4 morphant heads at 18 hpf (Student′s t-test, P<0.05). All scale bars equal 50 µm. Asterisks in A′ and C′ indicate the closing or fused choroid fissure in control morphants; brackets in B′ and D′ indicate the open choroid fissure in sox4 morphants. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Sox4 knockdown causes ectopic cell proliferation in the retina. (A) Quantification of PH3-positive cells in control and sox4 morphant retinas from 18 to 72 hpf. 8?10 individuals were analyzed in each time point (Studentós t-test, P<0.01). (B?K) Representative transverse sections through the optic vesicle and retina of control and sox4 morphants immunolabeled for PH3 at the indicated time points. Ectopic PH3-positive cells are indicated by arrows. At 72 hpf, the ectopic PH3-positive cells in the GCL also clustered with PCNA-positive cells (K, arrow). (L) Quantification of TUNEL-positive cells in control and sox4 morphant optic vesicle and retina from 18 to 72 hpf. 10?20 individuals were analyzed for each time point (Studentós t-test, P=0.017). (M?V) Representative transverse sections of control and sox4 morphant retinas labeled for TUNEL-positive cells from 18 to 72 hpf. (W) Quantification of TUNEL-positive cells in control and sox4 morphant optic stalk at 18 hpf. Arrows in N indicate increased TUNEL-positive cells in the optic stalk of an 18 hpf sox4 morphant. All scale bars equal 50 µm. PHENOTYPE:
|
|
Hh signaling is elevated in Sox4-deficent zebrafish. (A?D′) Representative eye and whole body images of vehicle-treated and cyclopamine-treated sox4 morphants at 24 and 48 hpf. (E) Quantification of the percentage of coloboma in vehicle- and cyclopamine-treated control and sox4 morphants (control morphants+vehicle: 1.14±0.96% coloboma, n=2/175; control morphants+cyclopamine: 0.49±0.79% coloboma, n=1/205; sox4 morphants+vehicle: 57.78±2.28% coloboma, n=104/180; sox4 morphants+cyclopamine: 15.87±2.20% coloboma, n=26/165; Fisherós exact test, P<0.0001). (F?I) Representative eye and body lateral images (F?G′) and transverse sections (H, I) of ptch2:EGFP(+/) transgenic embryos injected with control (F-F′, H) or sox4 MO (G-G′, I) at 12 hpf. (J) qPCR analysis of ptch2 expression in embryos injected with control or sox4 MO at 8, 10, and 12 hpf. Relative transcript abundance was normalized to level of atp5h (three biological replicates; Student′s t-test, P<0.01). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Sox4 negatively regulates ihhb expression. (A) ihhb and shha qPCR of mRNA from control and sox4 morphant whole embryos at 8, 10 and 12 hpf, and heads at 18 and 24 hpf. Relative transcript abundance was normalized to level of atp5h or gapdh. Y axis represents the average ratio of normalized sox4 morphant to control expression (three biological replicates; Studentós t-test, P<0.01). (B?G) Ihhb expression in control and sox4 morphants at 12 and 24 hpf detected by WISH. Representative images are presented from 15?20 individuals analyzed. Lateral images in B?E focus on the midline; therefore the eye is not visible. Arrows in B and C mark the ventral forebrain at the level of the optic vesicle. (H) Western blot analysis of protein lysates from control or sox4 morphant heads at 24 hpf. Two biological replicates were performed (sets 1 and 2) (I) qPCR of mRNA from vehicle- and cyclopamine-treated sox4 morphants (Studentós t-test, P<0.01). (J) Quantification of the proportion of embryos with coloboma at 48 hpf in sox4 morphants co-injected with two different doses of either ihhb MO or shha MO (sox4 MO alone: 51.24±2.7%, n=209/404; sox4 MO+0.5 ng/embryo ihhb MO: 19.33±2.52%, n=14/75; sox4 MO+1.0 ng/embryo ihhb MO: 10.67±2.08%, n=9/91; sox4 MO+2.0 ng/embryo of shha MO: 41.84±5.62%, n=53/125; sox4 MO+3.0 ng/embryo of shha MO, 34.39±5.03%, n=79/229) Fisherós exact test, P<0.0001 for sox4 MO+ihhb MO; P=0.1676 for sox4 MO+2.0 ng/embryo of shha MO; P=0.0001 for sox4 MO+3.0 ng/embryo of shha MO. f, forebrain; tel, telencephalon; di, diencephalon, hy, hypothalamus; mhb, midbrain?hindbrain boundary; n, notochord. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Overexpression of sox4 inhibits Hh ligand expression and causes cyclopia. (A?F) Representative images of embryos injected with control tdTomato or sox4 mRNA. (A-B) Representative images of the head were taken at 72 hpf from the ventral side. (C-F) Lateral views of body showing the somites. Insets in (C) and (D) were enlarged in (E) and (F). (G) Quantification of the percentage of cyclopia in embryos injected with different combinations of control and sox4 mRNA. 1.0 ng/embryo Td-tomato: 0.76±1.31%, n=1/108; 0.2 ng sox4a/b+0.6 ng Td-tomato/embryo: 9.35±1.17%, n=13/139; 0.3 ng sox4a/b+0.4 ng Td-tomato/embryo: 17.03±3.69%, n=11/67; 0.4 ng sox4a/b+0.2 ng Td-tomato/embryo: 25.56±2.20%, n=25/97. (H) qPCR analysis of ihhb and shha expression in control and sox4 overexpressing heads at 18 and 24 hpf. In sox4 mRNA injected embryos, ihhb expression was significantly decreased by 1.61-fold at 18 hpf (Studentós t-test, P<0.05) and 1.51-fold at 24 hpf (Studentós t-test, P=0.057). Shha expression was also significantly reduced following sox4 mRNA injection at both 18 and 24 hpf (by 1.82-fold and 3.57-fold, respectively; Student′s t-test, P<0.05). |
|
Synergistic effect of sox4 and sox11 knockdown on coloboma. (A?H) Representative images of embryos injected with control MO (A, E), a half-dose of sox4 MO (B, F), a half-dose of sox11 MO (C, G), and half-doses of sox4 and sox11 MOs (D, H). (A, E) Half the normal dose of sox4 MO caused a low incidence of mild coloboma. (C, G) Half the normal dose of sox11 MO resulted in low incidence of coloboma and lens malformations at 24 hpf (C, arrow). (D, H) Injection of a half-dose of both sox4 and sox11 MO significantly increased the incidence of coloboma, and the coloboma phenotype was more severe compared to sox4 or sox11 morphants alone. (I) Quantification of the proportion of embryos with coloboma at 48 hpf (half-dose sox4 MO, 11.05±3.15%, n=16/145; half-dose sox11 MO, 22.31±4.0%, n=16/71; half-dose of both sox4 and sox11 MO, 72.47±2.95%, n=51/70; Fisherós exact test, P=0.0001). (J) Sox11 can compensate for the loss of Sox4. Injection of sox11 mRNA into sox4 morphants significantly reduced the incidence of coloboma from 41.09±7.36% (n=113/275) to 10.14±3.09% (n=28/276; Fisherós exact test, P<0.0001). All scale bars equal 100 µm. |
|
Co-localization of sox4a/b with cell specific markers at 72 hpf. (A-B′′) FISH for sox4a and sox4b followed by immunostaining for the ganglion and amacrine cell marker HuC/D. Whereas sox4a did not co-localize with HuC/D (A-A′′), some sox4b-positive cells co-localized with HuC/D (B-B′′, arrows). (C-D′′) FISH for sox4a and sox4b followed by immunostaining for the horizontal cell marker Prox1. Sox4a did not co-localize with Prox1 (C-C′′); however some sox4b-positive cells in the outer half of the INL co-localized with Prox1 (D-D′′, arrows). (E-F′′) FISH for sox4a and sox4b followed by immunostaining for the bipolar cell marker PKC-α. No co-localization with PKC-α was detected. (G-H′′) FISH for sox4a and sox4b was performed in embryos transgenic for gfap:GFP which labels Müller glial cells. No co-localization with Müller cells was observed. All scale bars equal 25 µm. GCL, ganglion cell layer; INL, inner nuclear layer. EXPRESSION / LABELING:
|
|
Efficiency and specificity of sox4 MOs and sox4 CRISPR phenotype. (A-D′) EF1α-EGFP plasmids containing the sox4aor sox4b MO binding site were co-injected with either control MO or sox4 MO. At 24 hpf, control MO injected embryos showed ubiquitous GFP expression (A-B′), whereas sox4 MO injected embryos showed no GFP expression (C-D′). (E) Quantification of the percentage of injected embryos with GFP expression at 24 hpf. In control MO injected embryos, 91.94±1.93% had GFP expression (n=107/116). In sox4 MO injected embryos, none of them showed any GFP expression (n=0/227). (F) Sox4a and sox4b are both required for choroid fissure closure. Two different doses of sox4a and sox4b MO were injected into zebrafish embryos either separately or combined. A synergistic effect on the incidence of coloboma was observed when sox4a and sox4bMO were co-injected at both doses (Fisher′s exact test, P<0.0001). (G-J) A second set of non-overlapping sox4a/b MOs also resulted in coloboma at similar proportions to sox4 MO1 (40.85±8.39%, n=183/448). Bracket and arrows in (H) and (I) indicate the open choroid fissure and extrusion of the retina at the coloboma region. (K-L) Representative images of eyes from uninjected control (UIC) and tyrosinase (tyr)sgRNA/Cas9 injected embryos at 48 hpf. The tyr sgRNA/Cas9 injected embryos displayed a reduction in retinal pigmentation but did not have coloboma. (M-N) Representative images of eyes from sox4a and sox4bsgRNA/Cas9 injected embryos at 48 hpf that displayed coloboma (asterisk). (O) Quantification of the percentage of embryos with coloboma at 48 hpf. (P-Q) HRMA analysis detected the presence of mutant alleles in individual sox4a/b sgRNA/Cas9 injected embryos (arrows), which were confirmed by sequencing (not shown). |
|
Sox4 knockdown causes ectopic proliferation in the ganglion cell layer. (A-B) Clusters of PNCA- and PH3-positive cells were observed in the GCL of sox4 morphant retinas at 72 hpf (B, dashed line), whereas in control morphants proliferative cells were only observed in the CMZ. (C-D) Pax6a was expressed in the GCL and inner half of the INL in control and sox4 morphants at 72 hpf; however a dense clump of extra cells in the GCL of sox4 morphants was pax6a-negative (D, dashed line). (E-F) Immunostaining of control and sox4 morphant retinas with the ganglion cell marker Zn-8. Clusters of Zn-8-negative cells were present in the GCL of sox4 morphants (F, dashed line). D, dorsal; V, ventral; L, lens; CMZ, ciliary marginal zone; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; ON, optic nerve. |
Reprinted from Developmental Biology, 399(1), Wen, W., Pillai-Kastoori, L., Wilson, S.G., Morris, A.C., Sox4 regulates choroid fissure closure by limiting hedgehog signaling during ocular morphogenesis, 139-53, Copyright (2015) with permission from Elsevier. Full text @ Dev. Biol.