- Title
-
Wnt-Dependent Epithelial Transitions Drive Pharyngeal Pouch Formation
- Authors
- Choe, C.P., Collazo, A., Trinh, L.A., Pan, L., Moens, C.B., and Crump, J.G.
- Source
- Full text @ Dev. Cell
|
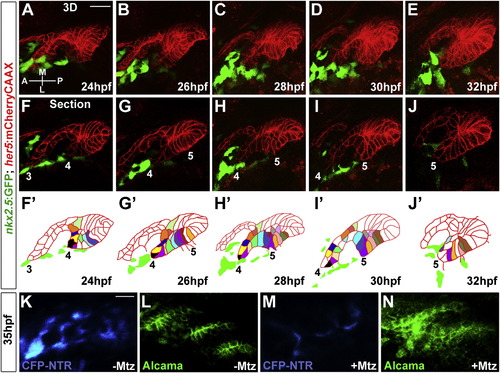
(F?J) Representative sections from the same time-lapse recording (see Movie S2) show various stages of development of pouches 3-5. In the schematics (F′?J′), the tracking of individually color-coded pouch cells highlights cell rearrangements. Cells that we could not track through the entire recording were left uncolored. |
|
Expression of wnt11r, wnt4a, and fzd8a during Pouch Formation(A?F) Colorimetric in situs show expression of wnt11r in discrete domains of mesoderm (arrowheads in A and B), wnt4a in ectodermal patches (arrowheads in C and D), and fzd8a in pouch-forming endoderm (E and F).(G?J) Double fluorescent in situs show colocalization of wnt11r but not wnt4a with nkx2.5 in lateral views (G and I) and higher magnification orthogonal sections (H and J, taken at level of white lines in G and I). Arrowheads indicate mesoderm and arrows ectoderm.(K) Fluorescent in situ shows colocalization of fzd8a (green) with her5-positive pouch endoderm labeled by GFP immunohistochemistry (red).(L) Schematic showing expression of wnt11r in mesoderm (blue), wnt4a in ectoderm (yellow), and fzd8a in endoderm (red) during pouch formation.(M?P) Compared to un-injected siblings (M and O), injection of 5 nl of 5 mM Mtz into nkx2.5:Gal4VP16; UAS:CFP-NTR embryos results in reduced numbers of mesodermal cells expressing nkx2.5 and wnt11r (arrowheads), as well as reduced ectodermal wnt4a expression. Scale bars, 20 μM. |
|
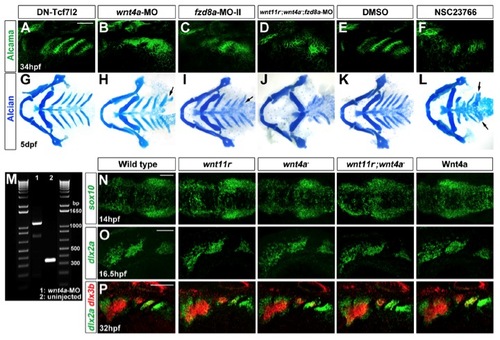
Roles of Wnt Signaling Components in Pouch and CB Cartilage Development(A?L) Alcama immunohistochemistry (green) shows defects in pouches in mutant, fzd8a-MO, and transgenic embryos. Identifiable pouches are numbered. UAS transgenic embryos (capitalized) were doubly positive for nkx2.3:Gal4VP16. Scale bar, 40 μM.(M?X) Whole-mount views of dissected facial cartilages. Arrows indicated fused or abnormal CB cartilages. Arrowhead indicates abnormal hyoid cartilage in fzd8a-MO embryos.(Y?AA) Superimposition of initial (blue) and final (red) still images from time-lapse recordings of fifth pouch development in wild-type, wnt11r-/-, and wnt4a-/- embryos. Colored lines indicate cell tracks (shown also below merged images), with filled circles denoting final positions.(AB) Average pouch cell speed in wild-types and mutants. Data represent mean ± SEM and p values are shown for each comparison.See also Figures S1 and S2 and Movies S3, S4, and S5. |
|
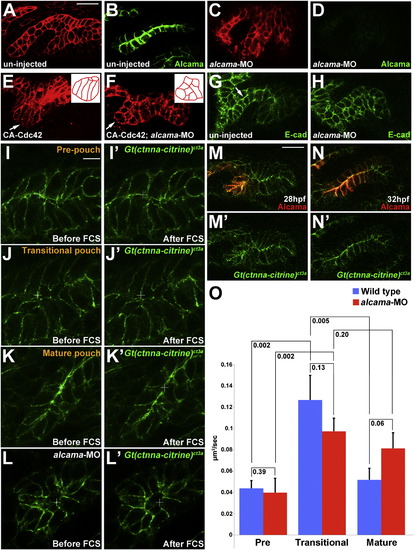
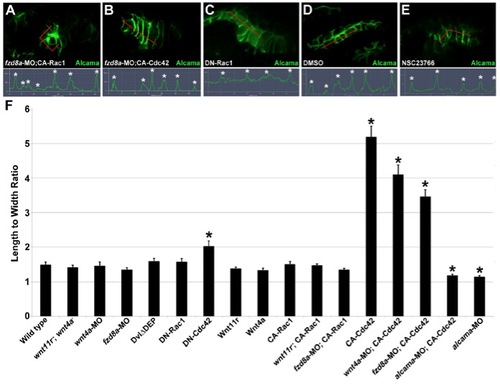
Wnt Signaling and Cdc42 Regulate Junctional Localization of Alcama in Developing Pouches(A?D) Immunohistochemistry shows progressive junctional localization of Alcama in the wild-type fourth pouch. Below each image, the normalized intensity of fluorescent signal is plotted along the 35 μM red lines. Asterisks indicate clear cell-cell boundaries.(E?H) Still images from a time-lapse confocal recording of fourth pouch development in nkx2.3:Alcama-GFP; her5:mCherryCAAX transgenic embryos (see Movie S6). As with endogenous Alcama protein, plots of fluorescence intensity through the red lines show progressive junctional localization of Alcama-GFP (green) within her5:mCherryCAAX-positive pouch cells (red).(I?X) Immunohistochemistry shows Alcama localization at the level of the fourth pouch in wild-type, mutant, and fzd8a-MO embryos, as well as UAS transgenic embryos (capitalized) doubly positive for nkx2.3:Gal4VP16. Alcama is partially mislocalized from pouch cell membranes to the cytoplasm in wnt4a-, wnt11r-wnt4a-, and fzd8a-MO embryos but not in wnt11r- embryos. In contrast, misexpression of Wnt11r results in disorganized endoderm (n = 28/32, data not shown) and occasionally rosette-like structures (n = 3/32), with rosette-like structures never observed in fzd8a-MO-injected siblings (n = 0/23, p = 0.003). Similarly, the inappropriate Alcama junctional localization induced by Wnt4a misexpression (n = 26/114) was never seen in fzd8a-MO-injected siblings (n = 0/74, p = 0.004) or doubly transgenic Wnt4a; DN-Cdc42 embryos (n = 0/31, p = 0.005). Rosette-like structures were observed in both CA-Rac1 embryos (n = 104/112) and their wnt11r mutant siblings (n = 11/17), and hyperelongated pouches were observed in both CA-Cdc42 embryos (n = 85/122) and their wnt4a-MO-injected siblings (n = 72/101). Plots of fluorescence intensity through the red lines are shown, with asterisks indicating clear cell-cell junctions.(Y?AB) Immunohistochemistry and normalized line plots show junctional localization of E-cadherin throughout wild-type pouch development, with pronounced apical enrichment (arrow in AA) as pouches mature. Inhibition of Dvl in nkx2.3:Gal4VP16; UAS:Dvl?DEP embryos does not significantly disrupt E-cadherin localization.See also Figure S3. EXPRESSION / LABELING:
PHENOTYPE:
|
|
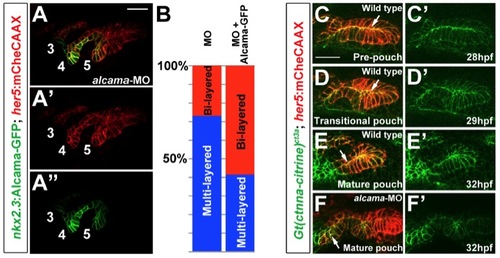
Alcama Is Required for Pouch Bilayer Formation and AJ Stabilization(A?D) her5:mCherryCAAX fluorescence (red) and Alcama immunohistochemistry (green) show loss of Alcama protein and an aberrant multilayered pouch morphology in alcama-MO embryos (C and D) compared to un-injected controls (A and B). Scale bar, 20 μM.(E and F) her5:mCherryCAAX labeling shows that the elongated morphology of pouch cells (arrows) resulting from CA-Cdc42 misexpression (n = 49/72) is suppressed by Alcama depletion (n = 0/67). Insets show schematics of pouch cell morphology.(G and H) Immunohistochemistry shows that E-cadherin still localizes to cell-cell junctions in the absence of Alcama protein yet the apical enrichment seen in wild-type mature pouches (arrow) is missing.(I?L) Imaging of α-catenin localization during three phases of wild-type pouch formation (I?K) and during a comparable phase of alcama-MO development (L) when wild-type pouches would have matured into bilayers. Crosshairs show target regions before FCS laser illumination (I?L) and 25 s after (I′?L′). Scale bar, 5 μM.(M and N) Alcama immunohistochemistry in wild-type Gt(ctnna-citrine)ct3a embryos shows that the appearance of Alcama (red) at apical and lateral cell-cell junctions corresponds to a transition from disorganized to strongly apical localization of α-catenin (green). Scale bar, 20 μM.(O) FCS measurements of endogenous α-catenin mobility in wild-type embryos show a significant increase in mobility from prepouch to transitional endoderm and a subsequent decrease in mature bilayers. In alcama-MO embryos, α-catenin mobility increases from prepouch to transitional endoderm but fails to decrease at a stage comparable to the mature wild-type pouch. n = 16 for each. Data represent mean ± SEM, and p values are shown for each comparison.See also Figure S4 and Movie S7. |
|
Wnt signaling in pouch, CB cartilage, and neural crest development, related to Figure 3. (A-L) Alcama immunohistochemistry (A-F) and Alcian Blue staining (G-L) show no pouch or CB cartilage defects upon PE-specific inhibition of nuclear-β-catenin signaling in nkx2.3:Gal4VP16; UAS:DN-Tcf7l2 embryos (A and G) or in control embryos incubated in 6% DMSO from 18-26 hpf (E and K). In contrast, severe pouch and CB defects were observed in wnt4a-MO (B and H), fzd8a-MO-II (C and I), and fzd8a-MO-injected wnt11r; wnt4a double mutants (D and J), as well as embryos incubated in 300 μM of the Rac inhibitor NSC23766 from 18-26 hpf (F and L). Arrows indicate fused or malformed CBs. |
|
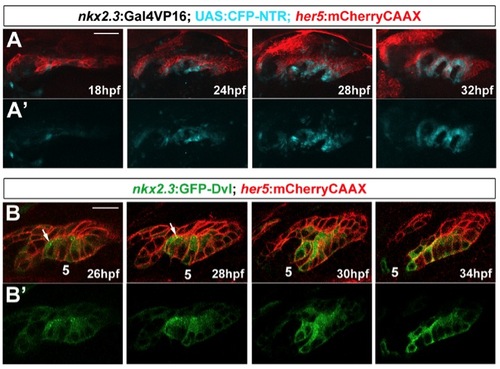
Specificity of nkx2.3:Gal4VP16 and GFP-Dvl localization in pouches, related to Figure 3. (A) Time-course of CFP-NTR expression in nkx2.3:Gal4VP16; UAS:CFP-NTR; her5:mCherryCAAX transgenic embryos. nkx2.3:Gal4VP16-dependent CFP-NTR expression (blue) co-localizes with her5:mCherryCAAX-positive pouch-forming endoderm (red) starting at 18 hpf, with expression becoming stronger as pouch development progresses. Views are dorsal-lateral with anterior to the left. Scale bar = 40 7mu;M. (B) Still images from a time-lapse confocal recording of fifth pouch development in nkx2.3:GFP-Dvl; her5:mCherryCAAX transgenic embryos (see Movie S5). GFP-Dvl (green) localizes to apical puncta (arrows) during remodeling of the her5:mCherryCAAX-positive endoderm (red) to form pouches. Scale bar = 20 μM. |
|
Alcama localization in rescued fzd8a-MO and Rac-inhibited embryos and quantification of cell shape, related to Figure 4. (A-E) Immunohistochemistry shows Alcama localization in 34 hpf pouch-forming endoderm in fzd8a-MO-injected nkx2.3:Gal4VP16; UAS:CA-Rac1 embryos (A), fzd8a-MO-injected nkx2.3:Gal4VP16; UAS:CA-Cdc42 embryos (B), nkx2.3:Gal4VP16; UAS:DN-Rac1 embryos (C), control embryos incubated in 6% DMSO from 18-26 hpf (D), and embryos incubated in the Rac inhibitor NSC23766 in 6% DMSO from 18-26 hpf (E). The normalized intensity of fluorescent signal is plotted along the 35 μM red lines. Asterisks indicate identifiable cell-cell boundaries. Activated Rac1 resulted in rosette-like structures in 59/81 fzd8a-MO-injected embryos, and activated Cdc42 rescued Alcama membrane localization and induced hyperelongated cell shape in 24/63 fzd8a-MO-injected embryos. (F) Quantification of 34 hpf pouch cell shape in wild type, mutants, fzd8a-MO, and embryos doubly transgenic for UAS transgenes (capitalized) and nkx2.3:Gal4VP16. The ratios of the longest versus shortest cell axes are plotted. Asterisks indicate significant differences compared to wild type (p<0.05 in a student?s t-test). Data represent mean ± SEM. Total cells analyzed: Wild type (96), wnt11r-; wnt4a- (100), fzd8a-MO (97), nkx2.3:Gal4VP16; UAS:DvlΔDEP (100), nkx2.3:Gal4VP16; UAS:DN-Rac1 (96), nkx2.3:Gal4VP16; UAS:DNCdc42 (92), nkx2.3:Gal4VP16; UAS:Wnt11r (45), nkx2.3:Gal4VP16; UAS:Wnt4a (100), nkx2.3:Gal4VP16; UAS:CA-Rac1 (92), fzd8a-MO; nkx2.3:Gal4VP16; UAS:CA-Rac1 (81), wnt11r-; nkx2.3:Gal4VP16; UAS:CA-Rac1 (48), nkx2.3:Gal4VP16; UAS:CA-Cdc42 (88), wnt4a-MO; nkx2.3:Gal4VP16; UAS:CA-Cdc42 (53), fzd8a-MO; nkx2.3:Gal4VP16; UAS:CACdc42 (57), alcama-MO; nkx2.3:Gal4VP16; UAS:CA-Cdc42 (60), and alcama-MO (95). |
|
Requirement of Alcama in endoderm and its role in α-catenin localization, related to Figure 5. (A) Transgenic expression of Alcama-GFP in nkx2.3:Alcama-GFP; her5:mCherryCAAX embryos partially restores bilayered pouch morphology to alcama-MO-injected embryos. Alcama-GFP (green) localizes to apical and lateral membranes of pouches labeled with her5:mCherryCAAX (red). Pouches 3-5 are numbered. Scale bar = 20 7mu;M. (B) Quantification of rescue of alcama-MO pouch defects by Alcama-GFP transgenic expression. Pouches 4 and 5 were used for the analysis. n=119 for alcama-MO + Alcama- GFP, and n=118 for alcama-MO alone. p-value = 0.000001. (C-E) Still images from a time-lapse confocal recording of fifth pouch development in a Gt(ctnna-citrine)ct3a; her5:mCherryCAAX embryo (See Movie S7). In the pre-pouch endoderm, &alpjha;-catenin-citrine (green) is enriched along apical membranes (arrows) of the her5:mCherryCAAX-labeled endodermal bilayer (red). During pouch initiation, α-catenincitrine becomes less organized in the multi-layered transitional epithelium. As pouches mature, α-catenin-citrine becomes organized again along apical membranes in the bilayer. (F) At a stage when wild-type pouches would have matured into bilayers, α-catenin-citrine remains disorganized in the alcama-MO multilayered pouches (arrow). |
Reprinted from Developmental Cell, 24(3), Choe, C.P., Collazo, A., Trinh, L.A., Pan, L., Moens, C.B., and Crump, J.G., Wnt-Dependent Epithelial Transitions Drive Pharyngeal Pouch Formation, 296-309, Copyright (2013) with permission from Elsevier. Full text @ Dev. Cell