- Title
-
BMP Signaling Modulates Hepcidin Expression in Zebrafish Embryos Independent of Hemojuvelin
- Authors
- Gibert, Y., Lattanzi, V.J., Zhen, A.W., Vedder, L., Brunet, F., Faasse, S.A., Babitt, J.L., Lin, H.Y., Hammerschmidt, M., and Fraenkel, P.G.
- Source
- Full text @ PLoS One
|
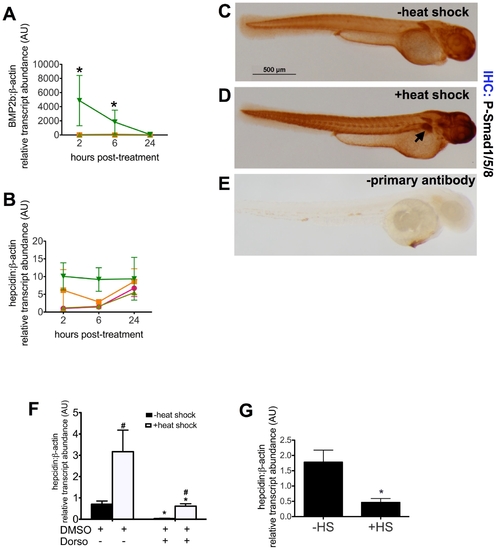
The BMP pathway regulates hepcidin expression in zebrafish embryos. A?B. Time course of bmp2b and hepcidin expression following induction of BMP2b expression. Tg(hsp70:bmp2b) is a transgenic line of zebrafish, which carries the BMP2b gene under the control of the hsp70 promoter. At 48 hours post-fertilization (hpf), WT or tg(hsp70:bmp2b) groups of embryos (n = 20 embryos per group) were subjected either to heat shock (+HS) at 37°C for 40 min or maintained at the usual temperature (28°C) (-HS). Pools of embryos were obtained for RNA extraction at 2, 6, and 24 hours after the start of heat shock, corresponding to 50, 54, and 72 hpf. Quantitative real-time RT-PCR was performed to measure transcript levels of bmp2b (A) or hepcidin (B), normalized to β-actin transcript levels and measured as fold increase over control, WT,-HS at 2 hours post-treatment. Data shown are means ± SE. * indicates p<0.05, compared to control. N = 2 pools per group. WT, -HS (pink circles), WT, +HS (orange squares), transgenic, -HS (light green triangles), transgenic, +HS (dark green triangles). C?E. Immunohistochemistry for P-Smad1/5/8. Compared to zebrafish embryos without BMP2b induction (C), P-Smad1/5/8 staining is increased in the liver (arrow) in tg(hsp70:BMP2b) embryos following heat shock (D). Omitting the primary antibody (anti-P-smad1/5/8), but including the biotinylated anti-Rabbit IgG/streptavidin horseradish peroxidase resulted in very low levels of background staining (E). N = 20 embryos per group. F,G. Inhibition of hepcidin expression by dorsomorphin (F) or noggin3 (G). F. From 28?55 hpf, pools of tg(hsp70:BMP2b) embryos were treated with the BMP inhibitor, 40 μM dorsomorphin (+Dorso), or treated with an equivalent amount of DMSO vehicle alone (+DMSO). Half the pools of embryos were subjected to heat shock at 48 hpf to induce bmp2b expression, followed by fixation at 55 hpf for quantitative real-time RT-PCR. G. Pools of embryos carrying tg(hsp70:noggin3) were subjected to heat shock or no heat shock at 48 hpf. The embryos were fixed at 55 hpf for quantitative real-time RT-PCR. Data shown are means ± SE. N = 4-5 pools per group. * indicates p<0.05, compared with no heat shock and no dorsomorphin treatment. # indicates p<0.05 compared with previous column. EXPRESSION / LABELING:
|
|
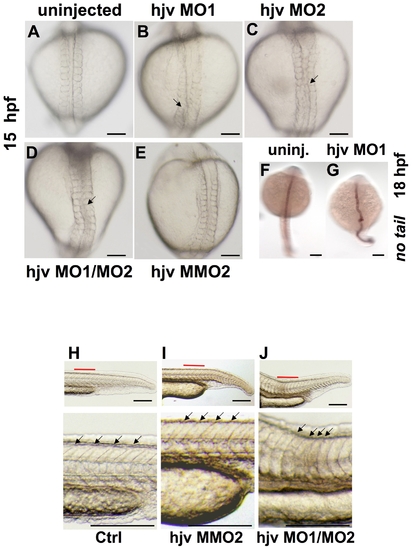
Morpholino knockdown of hjv results in notochord and somite abnormalities. A?E. Light microscopy of zebrafish embryos at 15 hpf in dorsal view Compared to uninjected embryos (A) or embryos injected with a mismatch control morpholino (E), embryos injected with either single hjv morpholinos (B, C) or a combination of hjv MO1 and hjv MO2 (D) exhibited a distorted notochord (arrows). N = 30 per group. F,G. Whole mount in situ hybridization at 18 hpf with no tail, which stains the notochord, illustrates the bent shape in the hjv MO1 injected morphants. N = 15 per group. H?J. Light microscopy of the tail at 24 hpf lateral view (top) with additional 3.5x enlargement of area labeled in red (below). Somites (arrows) in uninjected (H) and control morpholino-injected embryos (I) appeared V-shaped, while somites appeared U-shaped with decreased anterior-posterior dimension (distance between each pair of arrows) in hjv morphants (J). N = 20 per group. EXPRESSION / LABELING:
PHENOTYPE:
|
|
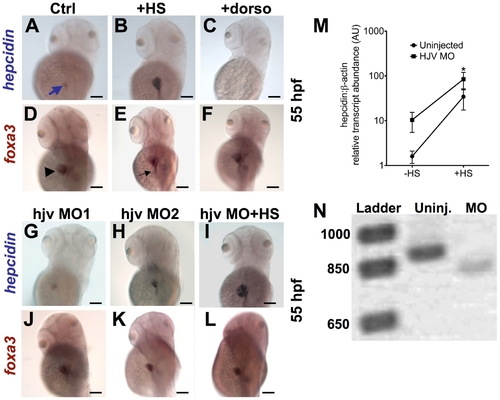
Knockdown of hjv does not significantly impair hepcidin expression at 55 hpf. A?L. Whole mount in situ hybridization at 55 hpf for hepcidin (blue arrow) (A?C, G?I) and foxa3 (D?F, J?L), as a marker for the liver (arrowhead) and intestine (black arrow). Compared to controls (A,D), induction of bmp2b by heat shock in tg(hsp70: bmp2b) embryos (B,E) increased hepcidin expression. Treatment with dorsomorphin from 28?55 hpf in WT embryos abrogated hepcidin expression, without affecting liver size (C,F). Knockdown of hjv by a morpholino blocking translation (G,J), or by a non-overlapping morpholino targeting a splice acceptor site (H,K), did not significantly change hepcidin expression, but slightly reduced liver size. Knockdown of hjv in tg(hsp70:bmp2b) embryos failed to prevent strong hepcidin expression following induction of bmp2b (I,L). N = 10?30 embryos per group. M. The effect of hjv knockdown on bmp2b-induced hepcidin transcript levels assessed by quantitative realtime RT-PCR. Embryos were injected with hjv MO2 at the one-cell stage followed by heat shock (HS) at 48 hpf and fixation for RNA extraction at 55 hpf. N. Electrophoresis of RT-PCR products, which were designed to amplify the targeted splice site, confirmed an 80 basepair alteration in transcript size, consistent with aberrant splicing of the hjv transcript in the morphants. |
|
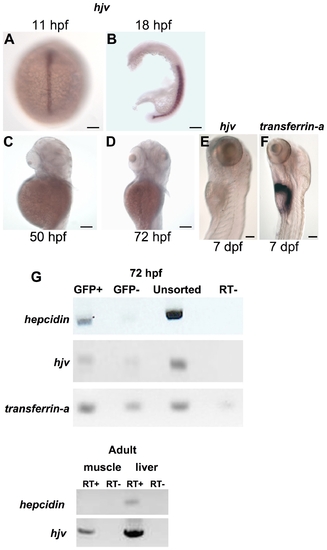
The hjv transcript is weakly expressed in the zebrafish embryonic liver at the time of hepcidin expression. A?F. Whole mount in situ hybridization for hjv (A?E) or transferrin-a (F) demonstrating expression in the notochord at 11 hpf (A) (dorsal view), in the somites at 18 hpf (B) (lateral view with yolk removed), but absence from the liver at 50 hpf (C), 72 hpf (D), and 7 days post-fertilization (dpf) (E). In comparison, transferrin-a is strongly expressed in the liver at 7 dpf (F). N = 10?30 embryos per group. G. Semiquantitative RT-PCR for hepcidin, hjv, and transferrin-a expression in embryonic zebrafish hepatocytes (top, GFP+) and nonhepatocytes (top, GFP-) and for hepcidin and hjv in adult zebrafish skeletal muscle and liver (bottom). Embryonic hepatocytes were sorted by FACS from pools of 80?100 transgenic zebrafish embryos at 72 hpf, which express GFP under the control of the liver-specific LFABP promoter. RT- indicates control reaction with reverse transcriptase omitted. EXPRESSION / LABELING:
|
|
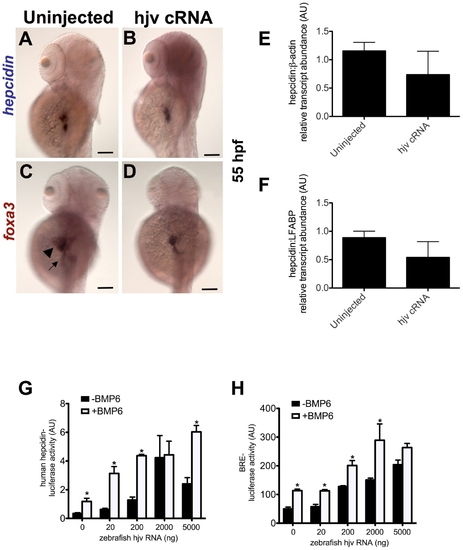
Overexpression of zebrafish hjv fails to increase hepcidin expression in zebrafish embryos, but can cooperate with BMP6 to activate the human hepcidin promoter in vitro. A?D. Whole mount in situ hybridization for hepcidin (A,B) or foxa3 (C,D) as a marker for the liver (arrowhead) and intestine (arrow) at 55 hpf following injection of zebrafish hjv cRNA at the one cell stage. N = 20?30 embryos per group. E,F. Quantitative real-time RT-PCR at 72 hpf demonstrated no significant change in hepcidin expression relative to β-actin (E) or to LFABP (F) following overexpression of hjv cRNA. N = 2 pools per group. G,H. In vitro luciferase reporter assays in Hep3B cells demonstrate the effect of increasing doses of zebrafish hjv cRNA on the human hepcidin promoter (G) or the BMP response element (H) in the absence (black) or presence (white) of exogenous BMP6 (5 ng/ml). Relative light units were calculated as ratios of Firefly (reporter) and Renilla (transfection control) values. Results from luciferase assay experiments were expressed as the means ± standard error of triplicates from representative experiments. * denotes p<0.05, compared to the previous column. |
|
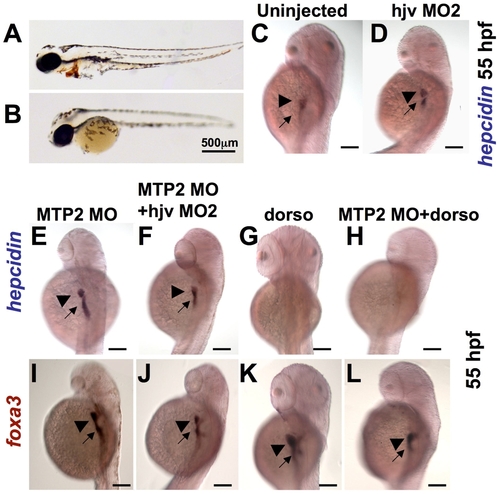
Knockdown of mtp2 enhances expression of hepcidin at 55 hpf. A?B. O-dianisidine staining for hemoglobin at 48 hpf demonstrated normal levels of hemoglobin in the cardiac circulation of WT embryos (A), but decreased hemoglobin in the mtp2 morphants (B). C?H. Whole mount in situ hybridization for hepcidin at 55 hpf demonstrated normal staining in uninjected controls (C) and hjv morphants (D). Knockdown of mtp2 (E) caused developmental delay, but increased the intensity of hepcidin staining in the liver (arrowhead) and the extent and intensity of staining in the intestine (arrow). Co-injection of hjv MO and mtp2 MO (F) resulted in a smaller embryo, but preserved hepcidin staining in the liver and intestine. Treatment with dorsomorphin from 28?55 hpf abrogated hepcidin expression in both uninjected embryos (G) and mtp2 morphants (H). I?L. Whole mount in situ hybridization for foxa3 demonstrated smaller liver size (arrowhead) in embryos injected with mtp2 MO (I,J,L), compared to dorsomorphin alone (K) or untreated embryos (compare with Figure 3D). N = 20?30 embryos per group. |
|
Knockdown of mtp2 increases hepcidin expression and iron staining in zebrafish embryos. A?F. Whole mount in situ hybridization at 72 hpf for hepcidin (dorsolateral) (A?C) and foxa3 (lateral) (D?F) in uninjected controls (A, D), compared to morphants of hjv (hjv MO2) (B,E) or mtp2 (C,F). Foxa3 marking the pharynx (blue arrow), liver (arrowhead), and intestine (black arrow) revealed a smaller liver size in hjv morphants (E) and particularly in mtp2 morphants (F). N = 40?45 embryos per group. G?I. Quantitative real-time RT-PCR for the liver specific marker, LFABP, relative to β-actin (G), and for hepcidin relative to the ubiquitous transcript, β-actin (H) or relative to LFABP (I). N = 2?8 pools of embryos per group. Data shown are means ± SE. * denotes p<0.05, compared to uninjected controls. J?Q. Whole mount nonheme iron staining of zebrafish embryos at 55 hpf with 5x additional magnification of boxed regions. We observed normal iron staining in uninjected WT (K) and hjv morphants (L), but increased iron staining (black arrows) in the somites and proctodeum (terminal gut) of hjv cRNA injected (M), mtp2 MO injected (N), erythroid transferrin receptor deficient mutant chianti (cia) (O), and in the dorsal spinal cord (blue arrows) of mtp2 morphants and chianti. As expected, decreased intraembryonic iron staining was observed in the transferrin-a deficient mutant gavi (gav) (P) and in the ferroportin deficient mutant weissherbst (weh) (Q). N = 11?20 embryos per group. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Treatment with dorsomorphin decreases BMP2b-induced phospho-smad1,5,8 staining in zebrafish embryos. Tg(hsp70:bmp2b) embryos were fixed at 55 hpf for immunohistochemical staining for phospho-smad1,5,8 following (A) no heat shock and no chemical treatment (-HS, -dorso), (B) no heat shock, but treatment with dorsomorphin (-HS, +dorso), (C) heat shock and no chemical treatment (+HS, -dorso), (D) heat shock and treatment with dorsomorphin (+HS, +dorso), representative embryos lateral view. Heat shock was performed at 48 hpf. Dorsomorphin treatment was performed from 28?55 hpf at a concentration of 40 μM. For enhanced sensitivity, a fluorescently-labeled secondary antibody was used (Alexa Fluor® 488 goat anti-rabbit IgG, Invitrogen, #A-11008). Embryos were illuminated with an X-cite Series 120 PC microscope lamp (Exfo Life Sciences and Industrial Division, Quebec, Canada) and emitted light was filtered with a green fluorescent protein (GFP) filter set. N = 15?22 embryos per group. |
|
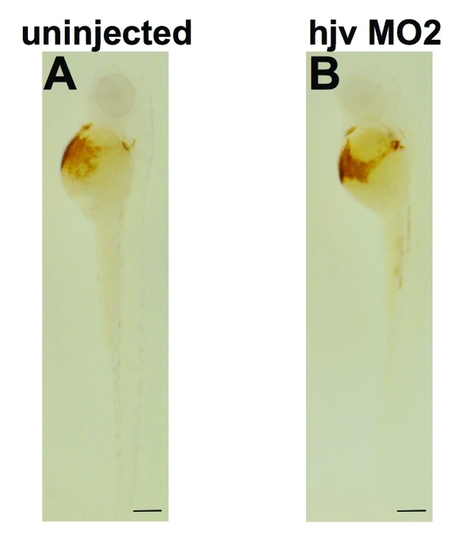
Knock down of hjv fails to produce anemia in zebrafish embryos. O-dianisidine staining for hemoglobin in embryos at 50 hpf, which were either uninjected (A) or injected with hjv MO2 (B) (lateral view). N = 42 embryos per group. |
|
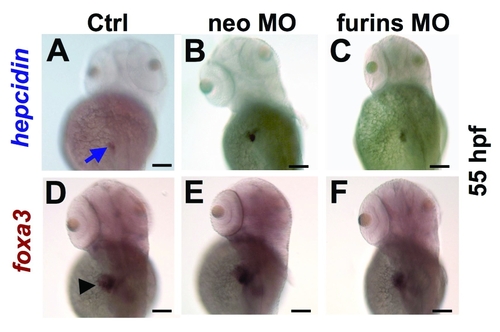
Knock down of hjv interacting proteins, neogenin or furin, fails to decrease hepcidin expression. Whole mount in situ hybridization for hepcidin (A?C, blue arrow) and foxa3 (D?F, black arrowhead) in uninjected embryos (A,D), compared to embryos injected with neogenin MO (B,E) or morpholinos directed against both zebrafish furins (furina and furinb) (C,F), dorsolateral view. N = 20 embryos per group. |
|
Neogenin knockdown reproduced the reported defect in somitogenesis associated with neogenin deficiency. A,B. Whole mount in situ hybridization for myoD to stain the somites in uninjected (A) and neogenin morphants (B) at the 20 somites′ stage of development (dorsal view) confirmed that injection of the neogenin morpholino at 0.15 mM produced elongation of the somites, manifest by increased distance between the two arrowheads. This is characteristic of the neogenin deficient phenotype, as described by [4]. Scale bar represents 100 microns. C,D. Whole mount in situ hybridization for hepcidin at 72 hpf in uninjected control embryos (C) and neogenin morphants (D) (lateral view) revealed a shortened body axis with a curved tail and flattened somites (arrowhead) in the neogenin morphants. Hepcidin expression is present in the liver (arrow) of the neogenin morphant, although the expression domain of hepcidin is smaller than in the uninjected control. Scale bar represents 200 microns. N = 20 embryos per group. Embryos were photographed at 100x magnification with a an Axio Imager 1 compound microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY) and an AxioCam ICc1 digital camera (Carl Zeiss MicroImaging, Inc.) (A,B) or a BX51 compound microscope (Olympus, Center Valley, PA) and a Q-capture 5 digital camera (QImaging, Surrey, BC, Canada) (C,D). PHENOTYPE:
|
|
Whole mount Alcian blue staining for cartilage in zebrafish embryos at 5 days post-fertilization confirms a branchial arch phenotype in furin morphants. Dorsolateral view of the head of an uninjected control embryo (A) and an embryo injected with morpholinos to knock down furina and furinb (B) reveals an open mouth phenotype (arrow in B) in the furina/furinb morphant. Lateral view of an uninjected control (C) and a furina/furinb morphant showing the fused cartilage elements (arrowhead in D) characteristic of furin morphants. N = 20 embryos per group. PHENOTYPE:
|
|
Phylogeny and expression of zebrafish RGM′s. Phylogenetic tree (A) of hjv and repulsive guidance molecule genes (RGM′s) in chordates. The four zebrafish RGM paralogs are highlighted in red. Hjv is also known as RGMc. B?I. Whole mount in situ hybridization of zebrafish embryos, dorsolateral views, at 50 hpf (B,D,F,H) and 72 hpf (C,E,G,I), for RGMa (B,C), RGMb (D,E), hjv (F,G), and RGMd (H,I) revealed that none of the RGM genes are detectable in the developing liver. Strong staining was detected in the mid and hindbrain for RGMa at 50 hpf (B) and 72 hpf (C, black arrows). At 50 hpf (D) and 72 hpf (E), RGMb is faintly expressed in the mid and hindbrain (black arrows). At 50 and 72 hpf, hemojuvelin is no longer detected in the developing embryo by in situ hybridization (F,G). At 50 hpf, RGMd transcripts were detected in the pharyngeal arches (H, black arrow). RGMd expression was no longer detected at 72 hpf (I). N = 20 embryos per group. (J) Phylogenetic tree of the RGM gene family constructed with all available vertebrate sequences. Note that hjv is expressed in a wide range of mammals, fish, and in Xenopus. We have identified hjv in the genome of a bird, the zebra finch (arrow), for the first time. RGMd has only been identified in fish. To generate the tree shown, we downloaded the protein sequences of the RGM gene families defined in the Ensembl database version 52 (as of December 2008) (<http://www.ensembl.org/>), which includes the hjv sequences. In addition to the Ensembl data, which also includes the Uniprot database (<http://www.uniprot.org/>), we also screened the NCBI database (<http://www.ncbi.nlm.nih.gov/>). Alignments were generated using ClustalW and Muscle[5], [6], followed by manual refinement using SeaView[7] to remove redundant and improperly annotated sequences. Phylogenetic tree reconstruction was carried out using the maximum likelihood (ML) method. Of note, the neighbor-joining (NJ) method[7] gives the same basal node topology. For ML analyses, robustness of the obtained tree topologies was assessed with 1000 bootstrap replicates; those below 50% are not shown. The NJ tree was constructed with Phylo_Win using a Poisson correction and pairwise gap removal[7]. The ML tree was obtained with PhyML[8] using a JTT model[9], a discrete gamma model with 4 categories. The gamma shape parameter was estimated by ML and the proportion of invariable sites was also estimated by ML. EXPRESSION / LABELING:
|
|
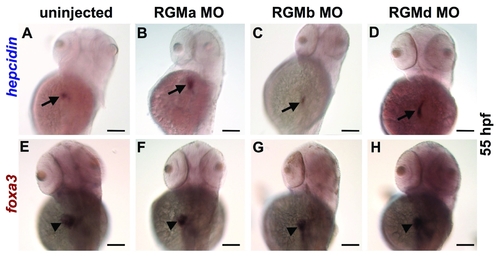
Effect of morpholino knockdown of RGM genes at 55 hpf. Whole mount in situ hybridization for hepcidin (A?D) or foxa3 (E?H), dorsolateral views. Compared to uninjected controls (A), knockdown of RGMa (B), RGMb (C), or RGMd (D) failed to inhibit hepcidin expression (arrow). E?H. Expression of foxa3 in the liver (arrowhead) revealed a slight reduction of liver size in the morphants (F?H) compared to control (E). N = 20 embryos per group. |
|
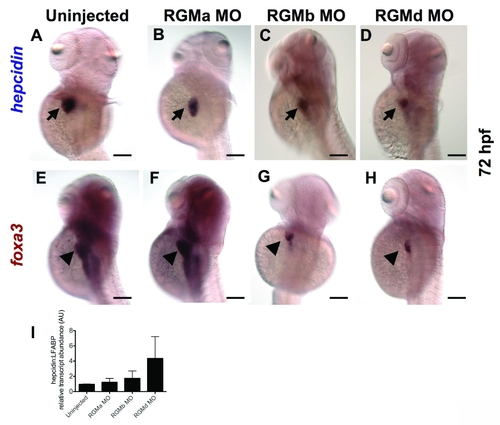
Effect of knockdown of RGM genes at 72 hpf. Whole mount in situ hybridization for hepcidin (A?D) or foxa3 (E?H), dorsolateral views. Compared to uninjected controls (A), knockdown of RGMa (B), RGMb (C), or RGMd (D) failed to inhibit hepcidin expression. E?H. Expression of foxa3 in the liver revealed a significant reduction of liver size in the RGMb and RGMd morphants (G, H). N = 20 embryos per group. I. Quantitative real-time RT-PCR revealed no significant decrease in hepcidin transcript levels relative to liver fatty acid binding protein (LFABP). N = 3 pools of embryos per group. Data shown are means + SE. EXPRESSION / LABELING:
PHENOTYPE:
|
|
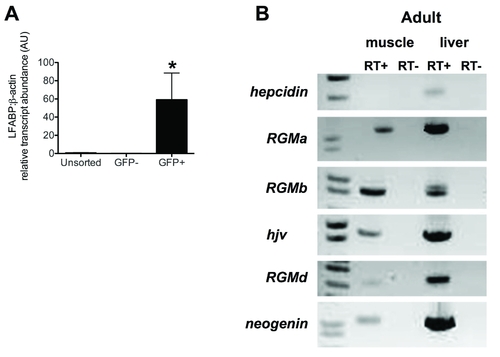
Additional expression data for zebrafish embryonic hepatocytes and zebrafish adult tissues. A. Quantitative real-time RT-PCR to assess transcript levels of LFABP (liver fatty acid binding protein) relative to β-actin in hepatocytes sorted from pools of 80?100 transgenic zebrafish embryos at 72 hpf. N = 2 pools per group. Data shown are means +/- SE. * indicates p<0.05 compared to unsorted. B. Semiquantitative RT-PCR for hepcidin, RGMa, RGMb, hjv, RGMd, and neogenin performed with RNA from adult zebrafish liver and skeletal muscle. Hepcidin expression was detected in the adult liver, but not in adult skeletal muscle. All RGM genes and neogenin were detected in the adult liver and skeletal muscle. |
|
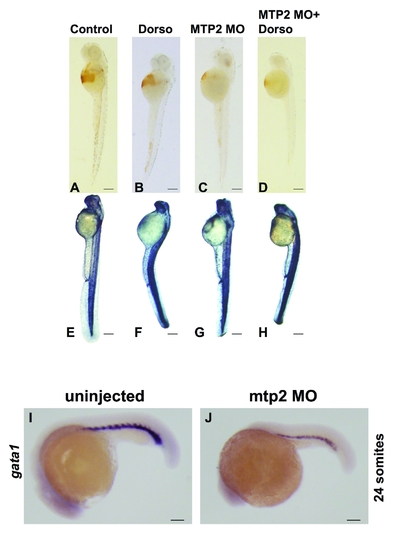
Effect of dorsomorphin on anemia and iron loading in mtp2 deficient embryos. Embryos were injected with mtp2 morpholino at the one cell stage, followed by treatment with dorsomorphin from 28 hpf until fixation for either o-dianisidine staining at 50 hpf (A?D) or whole mount nonheme iron staining at 55 hpf (E?H), lateral views. Uninjected controls (A) and embryos treated with dorsomorphin (B) exhibited normal hemoglobin staining, while mtp2 morphants (C) manifest decreased hemoglobin staining, which failed to improve when mtp2 morphants were treated with dorsomorphin (D). N = 54?99 embryos per group. Compared to uninjected controls (E), embryos treated with dorsomorphin (F), mtp2 morphants (G), or mtp2 morphants treated with dorsomorphin (H) exhibited increased iron staining in the somites, brain, and dorsal spinal cord. N = 32?45 embryos per group. (I,J) Whole mount in situ hybridization for gata1 (lateral views) when embryos have developed 24 somites, about 22 hpf, demonstrated decreased numbers of gata1-staining erythroid precursors in mtp2 morphants compared to uninjected embryos. N = 21?36 embryos per group. EXPRESSION / LABELING:
PHENOTYPE:
|

Unillustrated author statements PHENOTYPE:
|