- Title
-
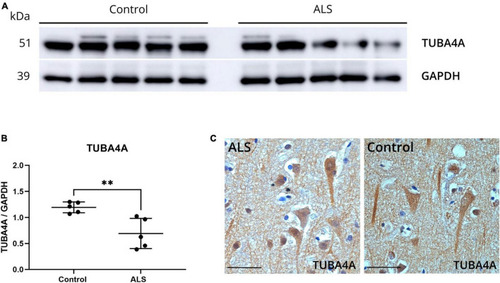
TUBA4A downregulation as observed in ALS post-mortem motor cortex causes ALS-related abnormalities in zebrafish
- Authors
- Van Schoor, E., Strubbe, D., Braems, E., Weishaupt, J., Ludolph, A.C., Van Damme, P., Thal, D.R., Bercier, V., Van Den Bosch, L.
- Source
- Full text @ Front. Cell. Neurosci.
|
ALS |
|
Specific |
|
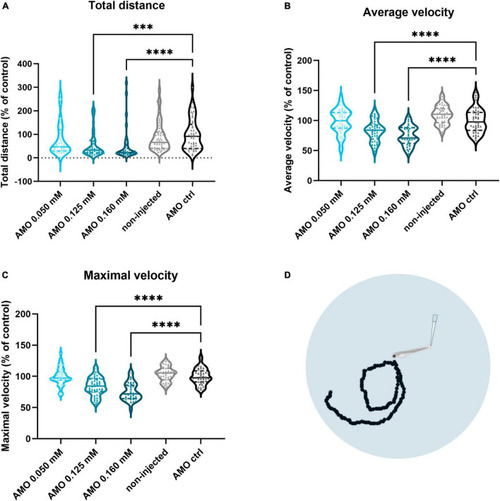
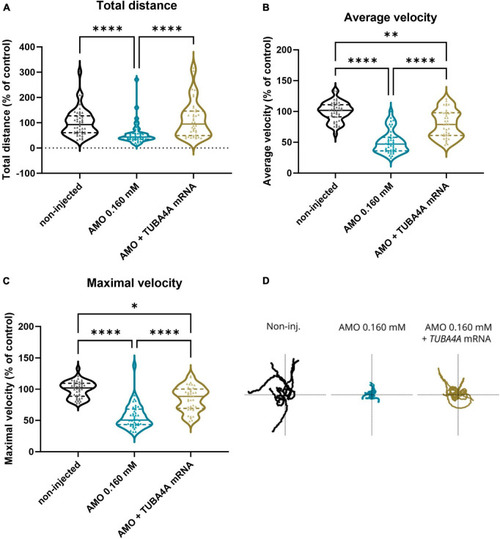
Zebrafish motor behavior deficits are induced by PHENOTYPE:
|
|
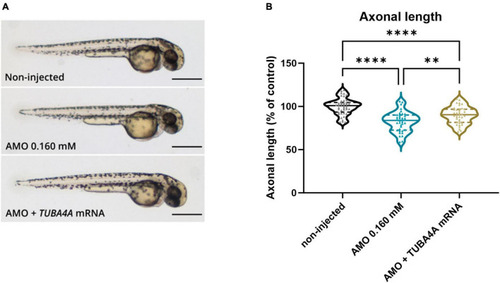
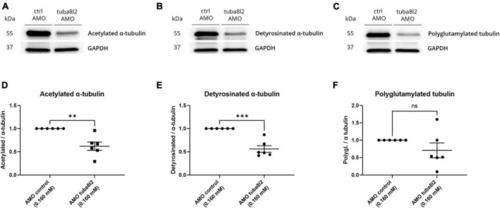
Rescue of axonal length defects by the addition of human wild-type PHENOTYPE:
|
|
Rescue of motor behavior deficits by the addition of human wild-type PHENOTYPE:
|
|
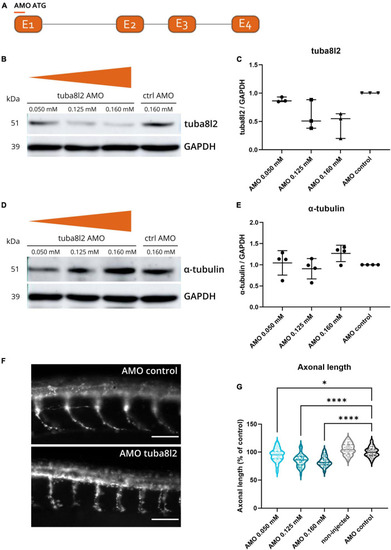
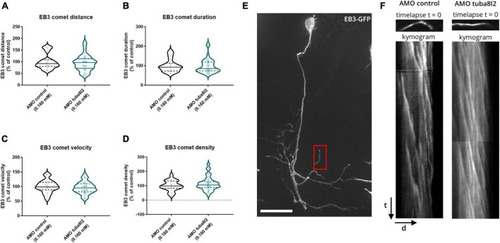
Zebrafish microtubule polymerization is not affected by |
|
Specific |