- Title
-
The Ciliopathy Gene cc2d2a controls Zebrafish Photoreceptor Outer Segment Development Through a Role in Rab8-dependent Vesicle Trafficking
- Authors
- Bachmann-Gagescu, R., Phelps, I.G., Stearns, G., Link, B.A., Brockerhoff, S.E., Moens, C.B., and Doherty, D.
- Source
- Full text @ Hum. Mol. Genet.
|
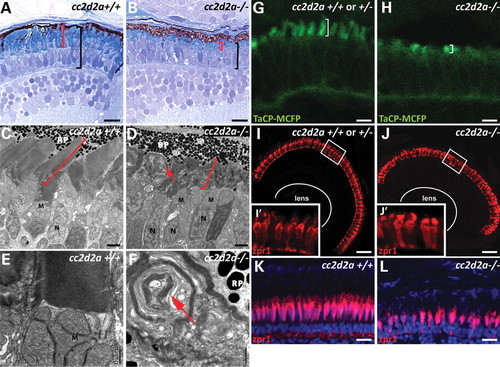
cc2d2a is required for zebrafish photoreceptor outer segment development. (A and B) Plastic sections of 5 d.p.f. cc2d2a+/+ (A) and cc2d2a-/- (B) retinas. Black brackets highlight the photoreceptor cell layer, and red brackets highlight the outer segments. (C?F) Transmission electron microscopy on 5 d.p.f. retinal sections shows disorganized stacks of membrane (red arrow) in the outer segments of cc2d2a-/- retinas (D) compared with wild-type (C). Higher power views (E and F) show whorls of membrane stacks replacing the outer segment (red arrow). (G and H) Tg(TaCP:MCFP) expression in 80 h.p.f. cryosectioned retinas. Wild-type cc2d2a+/+ or +/- cone photoreceptors have readily visible outer segments (G), whereas cc2d2a-/- photoreceptors have only short outer segments without the typical shape at the same stage (H). (I?J′) Red?green cones labeled with zpr1 antibody in wild-type (I and I′) and cc2d2a-/- (J and J′) 5 d.p.f. retinal cryosections showing comparable density of cone photoreceptors and grossly preserved photoreceptor cell morphology except in the apical segment. (K and L) Red?green cone cell bodies labeled with zpr1 antibody (red) in retinal cryosections from 4-week-old wild-type (K) and cc2d2a-/- fish (L). Fluorescent images are single-confocal sections. M, mitochondria; N, nuclei; RP, retinal pigment. Scale bars are 10 Ám in (A and B), 2 Ám in (C and D), 0.5 Ám in (E and F), 4 Ám in (G and H), 20 Ám in (I and J) and 10 Ám in (K and L). EXPRESSION / LABELING:
PHENOTYPE:
|
|
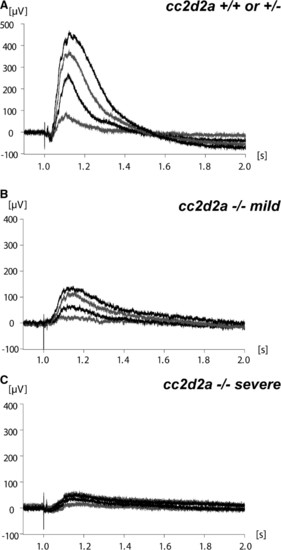
Visual function is abnormal in cc2d2a-/- zebrafish. (A) Representative ERG from cc2d2a+/+ or +/- 6 d.p.f. fish (n = 6). (B and C) ERGs from mildly (B) and severely (C) affected cc2d2a-/- 6 d.p.f. fish (n = 6). Different tracings in each graph represent different light intensities. PHENOTYPE:
|
|
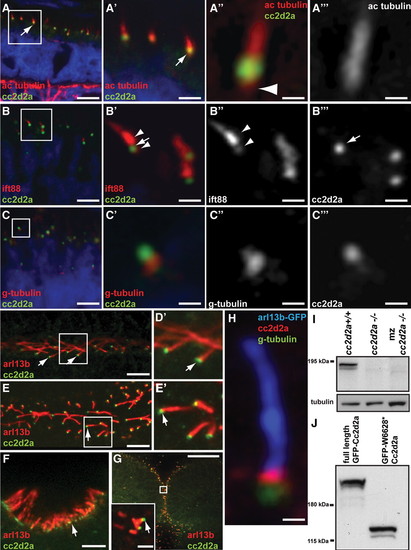
Cc2d2a localizes to the connecting cilium/transition zone of cilia in photoreceptors and other cell types. A?C′′′) Retinal cryosections from cc2d2a+/+ embryos labeled with anti-Cc2d2a (green) and anti-acetylated tubulin [red in (A?A′′′)], anti-Ift88 [red in (B?B′′′)] and anti-gamma-tubulin [red in (C?C′′′)] show Cc2d2a localization at the base of cilia (arrows). The Cc2d2a signal is apical to the basal-most acetylated tubulin signal [arrowhead in (A′′)], apical to the gamma-tubulin signal (C?C′′′) and within the Ift88-poor region that represents the connecting cilium, between the brightest Ift88 signal and a fainter, more basal signal [arrowheads in (B′ and B′′)]. Embryos are 4 d.p.f. in (A) and 5 d.p.f. in (B and C). (D?G) Immunofluorescence against Cc2d2a (green) and Arl13b (red) showing Cc2d2a localization at the base of 48 h.p.f. pronephric duct cilia (D and D′), 24 h.p.f. floorplate cilia (E and E′), 72 h.p.f. olfactory pit cilia (F) and 24 h.p.f. cerebellar neuronal progenitor cilia (G). (H) High power confocal image of a neuronal progenitor cilium from a 24 h.p.f. arl13b-GFP embryo stained with antibodies against gamma-tubulin (green), Cc2d2a (red) and GFP (blue). (D?H) Single-confocal sections of whole-mount embryos. Scale bars are 6 Ám in (A), 2 Ám in (A′), 0.4 Ám in (A′′ and A′′′), 4 Ám in (B and C), 1 Ám in (B′?B′′′), 0.5 Ám in (C′?C′′′), 5 Ám in (D and F), 10 Ám in (E), 20 Ám in (G) (2 Ám in inset) and 0.6 Ám in (H). (I) Western blot with Cc2d2a P2D9 antibody from whole wild-type, zygotic and maternal zygotic (mz) cc2d2a-/- embryo lysates at 48 h.p.f. The Cc2d2a band appears as a doublet around 190 kDa in wild-type (cc2d2a+/+) and is absent in zygotic and mz cc2d2a-/- embryos. (J) Western blot with Cc2d2a P2D9 antibody on full-length and truncated (W628*) Cc2d2a-GFP protein overexpressed in HEK293T cells. The antibody recognizes the full-length as well as the truncated Cc2d2a protein. EXPRESSION / LABELING:
|
|
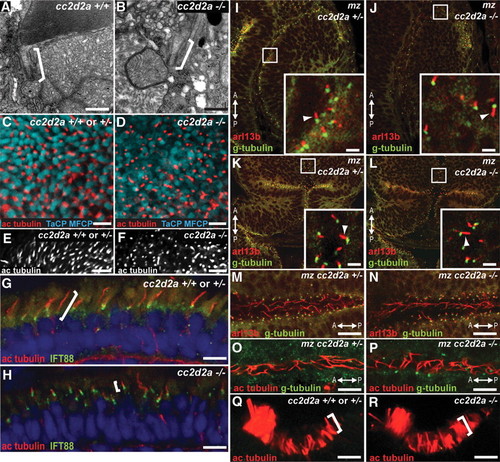
cc2d2a is not required for ciliogenesis. (A and B) Connecting cilia (brackets) in wild-type (A) and cc2d2a-/- (B) photoreceptors demonstrated by transmission electron microscopy. (C and D) Apical view of ciliary axonemes labeled with acetylated tubulin antibodies (red) in wild-type (C) and cc2d2a-/- (D) whole-mount eyes in which cone outer segments are labeled with tg(TaCP:MCFP). (E and F) Peripheral views of the same eyes as in (C and D) showing only the acetylated tubulin staining. Note the shorter axonemes in cc2d2a-/- eyes (F) compared with wild-type (E). (G and H) Ciliary axonemes labeled with acetylated tubulin (red) and Ift88 (green) antibodies in wild-type (G) and cc2d2a-/- (H) retinal cryosections. Brackets highlight axonemal length. Embryos are 5 d.p.f. in (A?F) and 7 d.p.f. in (G and H). (I?N) Immunofluorescence with Arl13b antibody (red) highlighting cilia and with gamma-tubulin antibody (green) highlighting basal bodies in 24 h.p.f. whole-mount mz cc2d2a+/- (I, K and M) and mz cc2d2a-/- (J, L and N) embryos showing retinal neuronal progenitor cilia (arrowheads in insets of I and J), cerebellar neuronal progenitors (arrowheads in insets of K and L) and floorplate cilia (M and N). (O and P) Pronephric duct cilia stained with acetylated tubulin (red) and gamma-tubulin (green) antibodies in 48 h.p.f. whole-mount mz cc2d2a+/- (O) and mz cc2d2a-/- (P) embryos. The anterior?posterior axis is indicated by the arrows. (Q and R) Acetylated tubulin antibody staining of olfactory pit cilia at 3 d.p.f. in whole-mount wild-type (Q) and cc2d2a-/- (R) embryos. (I?L) and (Q and R) Single-confocal sections. (C?F) and (M?P) Projected stacks of confocal sections. Scale bars are 500 nm in (A and B), 5 Ám in (C and D), 10 Ám in (E and F), 4 Ám in (G and H), 2 Ám in (I?L), 10 Ám in (M and N) and 5 Ám in (O?R). EXPRESSION / LABELING:
|
|
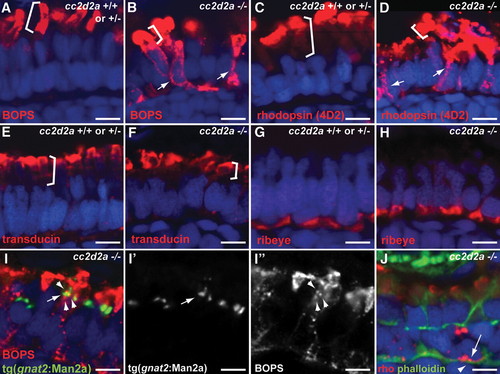
Selective trafficking defects in cc2d2a-/- photoreceptors. (A and B) BOPS localization (BOPS antibody, red) in wild-type (A) and cc2d2a-/- (B) photoreceptors. (C and D) Rhodopsin localization (4D2 antibody, red) in wild-type (C) and cc2d2a-/- (D) photoreceptors. Outer segments are indicated by brackets. Mislocalized photopigment (opsin) is indicated by white arrows. (E and F) Transducin (red) localization by immunofluorescence is restricted to the outer segments (indicated by brackets) in both cc2d2a+/+ and cc2d2a-/- photoreceptors. (G and H) Ribeye (red) localization at the synapse is indistinguishable between cc2d2a+/+ and cc2d2a-/- photoreceptors. (I-I′′) BOPS staining [red in (I)] and Golgi expression of tg(gnat2:Man2a-RFP, green) do not significantly overlap [see arrowheads in (I) and (I′′) for BOPS and arrow in (I) and (I′) for Golgi]. (J) Rhodopsin (4D2 antibody, red, arrow) is mislocalized between the nucleus (DAPI, blue) and the subcortical actin network stained with phalloidin (green, arrowhead). All images are single-confocal sections of 5 d.p.f. cryosections. Scale bars are 4 Ám in all panels. |
|
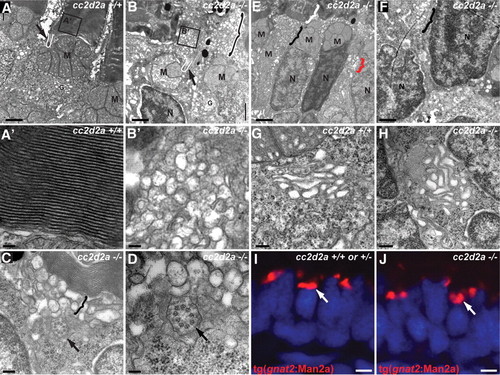
Loss of Cc2d2a leads to vesicle accumulation in photoreceptors. (A?H) Transmission electron microscopy images of 5 d.p.f. cc2d2a+/+ (A?A′ and G) and cc2d2a-/- (B?F and H) photoreceptors. (A) Low-power image of the inner segment and the base of the outer segment in cc2d2a+/+ photoreceptors showing the mitochondrial cluster M, the connecting cilium (arrow and white line) and the base of the outer segment (A′), with neatly stacked membranes [high power view (A′)]. (B) Low-power image of cc2d2a-/- photoreceptors with normal mitochondrial clusters M and nuclei N, but massive accumulation of vesicles around a normal-appearing connecting cilium [arrow and white line in (B)]. The black and white bracket highlights an outer segment replaced by vesicles. (B′) High-power image of the vesicles boxed in (B). (C and D) Higher power images of the accumulating vesicles (bracket) just below partially stacked membranes. (D) Cross-section through a connecting cilium (arrow) with nine normal appearing microtubule doublets. (E) Lower power image of a different region showing moderate vesicle accumulation (black bracket) below a recognizable outer segment (white bracket). Note the presence of vesicles lateral to the nucleus (red bracket). (F) Higher power image of the basal portion of a photoreceptor showing vesicle accumulations (bracket) between nuclei. (G and H) High-power images of the Golgi apparatus in wild-type (G) and cc2d2a-/- (H) photoreceptors. (I and J) Cryosections of transient transgenic cc2d2a+/+ (I) and cc2d2a-/- (J) embryos expressing the Golgi specific transgene tg(gnat2:Man2a)-RFP (red) under the transducin promoter control. Scale bars are 1 Ám in (A?B), 100 nm in (A′ and B′), 200 nm in (C), 100 nm in (D), 2 Ám in (E), 1 Ám in (F), 500 nm in (G and H) and 2 Ám in (I and J). M, mitochondria; N, nuclei; G, Golgi. |
|
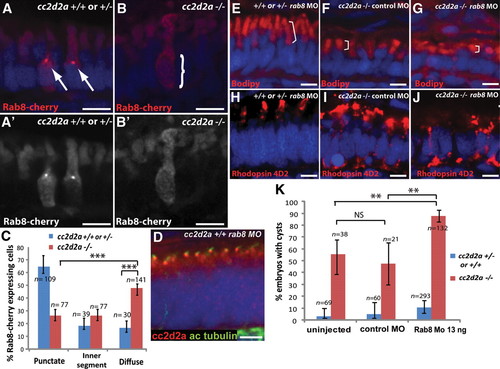
cc2d2a is required for Rab8 localization and interacts genetically with rab8 in ciliated cells. (A?B′) Expression of a transducin-promoter-driven Rab8-cherry construct in cc2d2a+/+ photoreceptors (A and A′) is concentrated in one punctum at the base of the outer segment (arrows), whereas it is diffuse in cc2d2a-/- photoreceptors (B and B′, bracket). (C) Proportion of Rab8-cherry expressing photoreceptors with punctate versus slightly enhanced inner segment or diffuse cellular expression (***P < 0.0001, χ2 test, bars indicate 95% confidence interval). (D) Cc2d2a localization (red) in 4 d.p.f. Rab8 morphant (rab8MO) retinas co-stained with acetylated tubulin to mark cilia. (E?J) Synergistic effect of partial rab8 knockdown on the retinal phenotype of cc2d2a-/- embryos. (E?G) Length of outer segments highlighted with bodipy counterstain (red, brackets) is reduced in rab8-morpholino-injected cc2d2a mutant embryos (G) compared with control-morpholino-injected mutants (F). At the same dose of rab8 morpholino, cc2d2a+/+ embryos have well-formed outer segments of normal length (E). (H?J) Rhodopsin localization (4D2 antibody, red) is virtually absent from remnant outer segments in rab8-morpholino-injected cc2d2a mutants (J) compared with control-morpholino-injected mutants (I). At the same dose of rab8 morpholino, there is no mislocalization of rhodopsin in cc2d2a+/+ siblings (H). (K) Histogram showing the proportion of embryos with pronephric cysts (red bars indicate cc2d2a-/- mutants and blue bars indicate wild-type and heterozygote siblings). Bars indicate 95% confidence intervals. Pairwise comparisons between rab8-morpholino-injected cc2d2a-/- fish and uninjected siblings, as well as rab8-morpholino-injected cc2d2a-/- and control (gbx2) morpholino-injected cc2d2a-/- fish are significant (**P = 0.0001, χ2 test). NS, non-significant. All images are cryosections of 5 d.p.f. retinas except (D), which is a cryosection of a 4 d.p.f. retina. Scale bars are 4 Ám in all panels. +/+ or +/- in (E) and (H) refers to cc2d2a+/+ or +/-. |
|
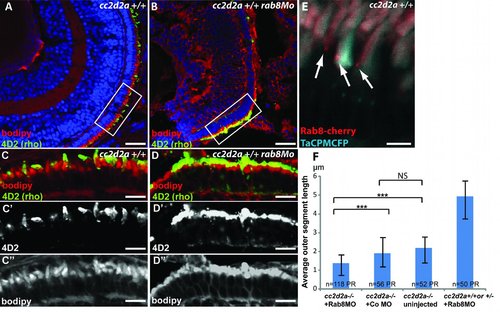
rab8 morphant phenotype and Rab8 localization at the base of the outer segment. (A-B) 4 dpf retinal cryosections of wild-type (A) and rab8 morphant (rab8MO)(B) embryos showing shortened outer segments stained with bodipy (red) and rhodopsin (4D2 antibody, green). (C-D) Higher power images of boxed areas in A and B with separate channel images for 4D2 (C′-D′) and bodipy (C′′-D′′) showing shortened and dysmorphic outer segments as well as rhodopsin mislocalization. (E) Rab8-cherry expression (red) in cryosections from wild-type TaCP:MCFP-expressing photoreceptors (outer segments are light blue) showing punctate localization at the base of the outer segment. Scale bars are 40 Ám in A-B, 10 Ám in C-D′′ and 4 Ám in E. (F) Average outer segment length in cc2d2a -/- embryos injected with Rab8MO, injected with a control MO, uninjected and cc2d2a +/+ or +/- embryos injected with Rab8Mo. Scale bars are standard deviations. *** p<0.0001, NS non significant (p=0.7, Chi-square test). PR photoreceptors. |