- Title
-
Inactivation of serine protease Matriptase1a by its inhibitor Hai1 is required for epithelial integrity of the zebrafish epidermis
- Authors
- Carney, T.J., von der Hardt, S., Sonntag, C., Amsterdam, A., Topczewski, J., Hopkins, N., and Hammerschmidt, M.
- Source
- Full text @ Development
|
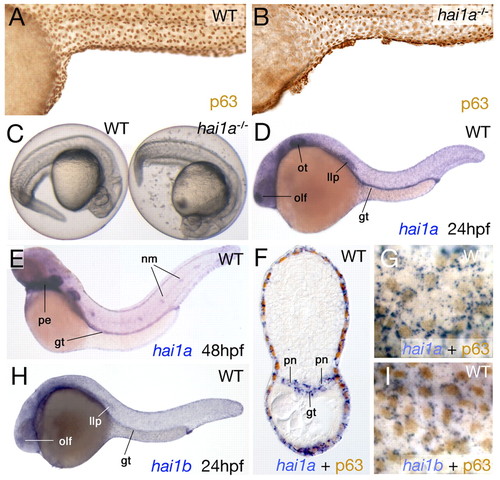
hai1a is expressed in basal keratinocytes and is required for the proper development of the epidermis. (A,B) Lateral views of the trunk of a wild-type sibling (WT; A) and a homozygous hi2217 embryo (hai1a-/-; B) at 48 hpf, after anti-p63 immunolabeling of basal keratinocytes. (C) Shedding of cells into the chorion is evident in hi2217 homozygotes (right embryo), compared with no shedding in the wild-type sibling (left embryo) from 24 hpf. (D,E,H) In situ hybridization showing the expression of hai1a at 24 hpf (D) and 48 hpf (E), and of hai1b at 24 hpf (H). The hai1a and hai1b expression domains in olfactory epithelium (olf), otic epithelium (ot), gut (gt), lateral line primordium (llp), neuromasts (nm) and pharyngeal endoderm (pe) are indicated. (F,G,I) Cross-section through the posterior trunk of a 24 hpf wild-type embryo after double staining for hai1a mRNA (blue) and p63 protein (brown) (F), and magnified views of the trunk epidermis of a 24 hpf wild-type embryo after double staining for hai1a (G) or hai1b (I) (blue) and p63 protein (brown). In F, the hai1a expression domains in the pronephric ducts (pn) and gut (gt) are indicated. |
|
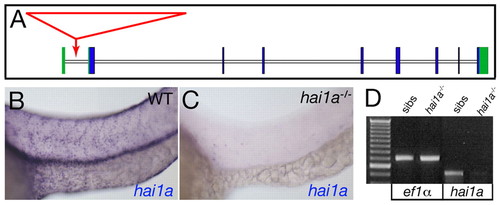
The insertion in hi2217 abrogates hai1a transcription. (A) Structure of the hai1a gene showing the viral insertion site in the hi2217 allele (red). Exons are boxed with coding and non-coding sequences in blue and green, respectively. The viral insertion (red arrow) occurs in the first intron upstream of the first coding exon. (B,C) Lateral views of the trunk epidermis of a wild-type sibling (WT; B) and a hi2217 homozygote (C) at 24 hpf, after hai1a in situ hybridization. (D) Reverse transcriptase (RT)-PCR analysis of hi2217 homozygotes (lanes 3,5) and wild-type siblings (lanes 2,4) at 24 hpf, demonstrating a strong reduction in hai1a transcript levels in mutants compared with siblings, whereas ef1a levels are identical. Lane 1, 100 bp ladder. |
|
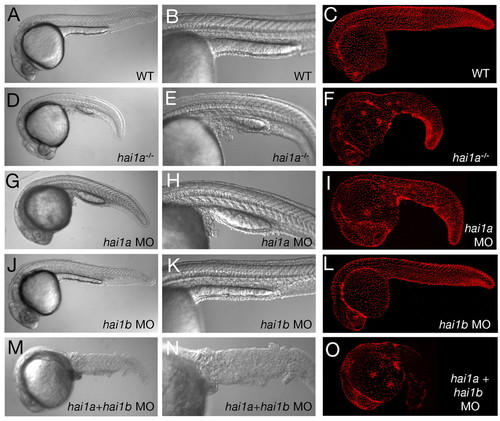
Keratinocyte aggregation and shedding caused by the loss of Hai1a is further enhanced by the concomitant loss of its paralog, Hai1b. All panels show embryos at 24 hpf; wild-type siblings (WT; A-C), hai1a mutants (D-F), hai1a morphants (G-I), hai1b morphants (J-L) and hai1a mutants injected with hai1b MOs (M-O). Shown are lateral views of live embryos using Nomarski optics at low power (A,D,G,J,M) to assess overall embryo morphology, and at higher magnification (B,E,H,K,N) to assess epidermal defects in the trunk/tail regions, and lateral views of embryos after immunofluorescent detection of the basal epidermal marker protein p63 (C,F,I,L,O; merged stacks of confocal images). PHENOTYPE:
|
|
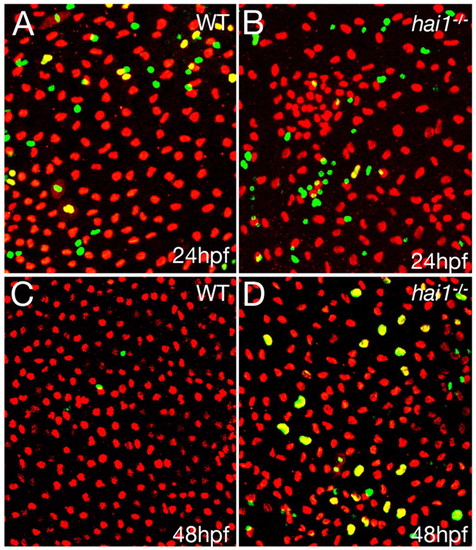
Enhanced basal keratinocyte proliferation in hai1a mutant embryos occurs subsequent to epidermal cell aggregation. (A-D) Confocal images of ventral yolk epidermis of wild-type siblings (WT; A,C) and hai1a mutants (B,D) at 24 hpf (A,B) and 48 hpf (C,D), after BrdU labeling of proliferating cells (green) and anti-p63 immunolabeling of basal keratinocytes (red). PHENOTYPE:
|
|
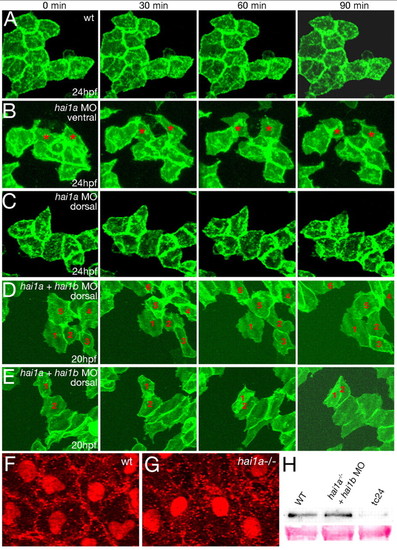
Loss of Hai1 activity abrogates the epithelial properties of basal keratinocytes. (A-E) Stills of in vivo time-lapse recordings of clusters of basal keratinocytes labeled with membrane-bound GFP, at the indicated times after 24 hpf (A-C) or 20 hpf (D,E); wild-type sibling (wt; A; see Movie 1 in the supplementary material); hai1a morphants (B,C; see Movies 2 and 3 in the supplementary material); and hai1a + hai1b double morphants (D,E; see Movies 4 and 5 in the supplementary material). Epidermal cells in the ventral regions of a hai1a morphant (B) display a mesenchymal-like behavior (examples indicated by asterisks; recorded region indicated in Fig. 6G). By contrast, epidermal cells in more-dorsal/posterior regions (C) form a rigid epithelium, as in wild-type embryos (A). In embryos lacking both Hai1a and Hai1b, motility and fibroblastoid behavior of basal keratinocytes is further enhanced, evident much earlier and now also seen in dorsal trunk/tail regions (D; individual keratinocytes labeled with numbers). Often, cells migrate on top of each other (E; cells 1 and 2). (F,G) Ventral confocal views of yolk epidermis of a 24 hpf wild-type sibling (F) and a hai1a mutant embryo (G), with immunofluorescent detection of E-cadherin (E-cad) and p63, marking nuclei of basal keratinocytes. (H) Anti-E-cad western blots of embryonic extracts from 24 hpf wild-type siblings (left lane) and hai1a mutants injected with hai1b MO (middle lane), revealing unaltered E-cad protein levels in double-deficient embryos. By contrast, offspring of smad5dtc24 heterozygous mothers (Hild et al., 1999), which, according to anti-p63 immunostainings, lack basal keratinocytes (data not shown), display strongly reduced E-cad protein levels (right lane). EXPRESSION / LABELING:
PHENOTYPE:
|
|
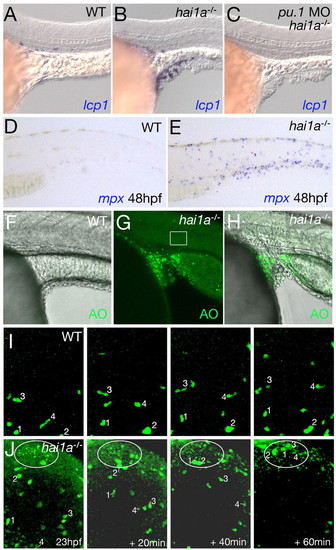
Skin inflammation in hai1a mutants is linked to keratinocyte death, but is dispensable for defects in epithelial integrity. (A-C) Lateral views of yolk sac extension of 24 hpf embryos, after in situ hybridization for the leukocyte marker lcp1. Leukocytes accumulate around epidermal aggregates in hai1a mutants (B); this does not occur in wild-type siblings (WT; A). hai1a mutants injected with pu.1 MO lack leukocytes but display epidermal aggregates of equal severity (C). (D,E) Lateral trunk views of a wild-type sibling embryo (D) and a hai1a mutant (E) after in situ hybridization for the neutrophil marker mpx at 48 hpf. (F-H) Lateral views of yolk sac extension of a 24 hpf sibling embryo (F) and hai1a mutant (G,H), stained with acridine orange (AO). (F,H) Overlays of fluorescent and Nomarski images; (G) fluorescent image. Box in G marks the site where Movie 2 in the supplementary material was taken, stills of which are shown in Fig. 5B. (I,J) Stills from time-lapse Movies 6 and 7 in the supplementary material (at the indicated times after 23 hpf), demonstrating that Tg(fli1a:egfp)-marked leukocytes of hai1a mutants migrate from their site of origin anterior to the cardiac field over the yolk sac (Herbomel et al., 1999) to sites with nascent epidermal aggregates and numerous apoptotic cells (J). A cluster of acridine orange (AO)-positive cells is outlined. By comparison, leukocytes of wild-type siblings move more slowly and in a less directed fashion (I). For tracking, four individual leukocytes are indicated with numbers. |
|
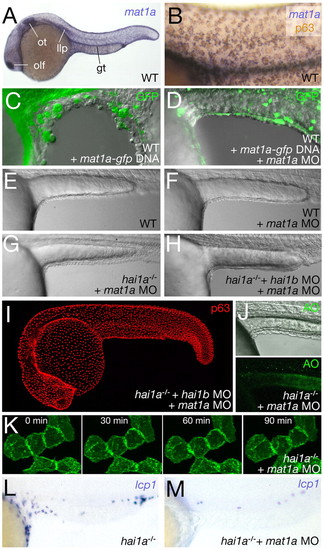
The defects of hai1 mutants are phenocopied by overexpression and rescued by knockdown of matriptase1a. (A) In situ hybridization revealing matriptase1a (labelled mat1a) expression in the epidermis, olfactory epithelium (olf), otic epithelium (ot), gut (gt) and lateral line primordium (llp) at 24 hpf. (B) Counter-staining with p63 antibody (brown), demonstrating expression in the basal epidermal layer. (C,D) Lateral views of yolk sac extension of heat-shock-treated embryos injected with pTol2-hse-GTP/matriptase1a alone (C), displaying epidermal dissociation, or co-injected with pTol2-hse-GTP/matriptase1a and matriptase1a MO (D), displaying normal epidermal morphology; 24 hpf, overlays of fluorescent and Nomarski images. Cells with transgene expression are labeled by GFP. (E,F) Lateral Nomarski images of a 24 hpf un-injected wild-type embryo (E) and matriptase1a morphant (F), demonstrating that the loss of Matriptase1a does not affect epidermal morphology. (G,H) Lateral Nomarski images of hai1a mutants injected with either matriptase1a MO alone (G) or with matriptase1a and hai1b MOs (H); both display normal epidermal morphology. (I) Anti-p63 immunostaining of a 24 hpf hai1a mutant co-injected with matriptase1a and hai1b MOs. (J) Acridine orange (AO) staining alone (lower panel) and super-imposed with a Nomarski image (upper panel) of the yolk extension region of a 24 hpf hai1a mutant injected with matriptase1a MO. (K) Stills of time-lapse Movie 8 in the supplementary material (at the times indicated after 24 hpf), demonstrating that matriptase1a MO injection restores the epithelial properties of fluorescently labeled hai1a morphant basal keratinocytes. (L,M) Lateral views of the trunk and tail of an un-injected hai1a mutant (L) and of a hai1a mutant injected with matriptase1a MO (M) after in situ hybridization for the leukocyte marker lcp1 at 24 hpf. EXPRESSION / LABELING:
|
|
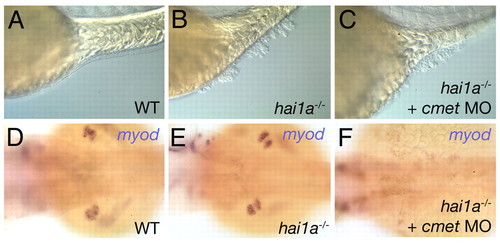
Inactivation of Met fails to rescue epithelial defects in hai1a mutants. (A-C) Lateral Nomarski images of a wild-type sibling (WT; A), hai1a mutant (B) and met (cmet) MO-injected hai1a mutant (C) at 48 hpf, revealing the presence of epidermal aggregates both in injected (C) and un-injected (B) mutants. (D-F) Dorsal views of the trunk region of embryos shown in A-C, with the pectoral fin muscle labeled for myoD transcripts. The pectoral fin muscle is absent from the met morphant (F), compared with un-injected siblings (D,E), demonstrating that the MO is functional. Notice the presence of normal pectoral fin muscle in the hai1a mutant (E), indicating that Hai1a is dispensable for the Hgf- and Met-dependent migration of fin muscle precursor cells (Haines et al., 2004). |
|
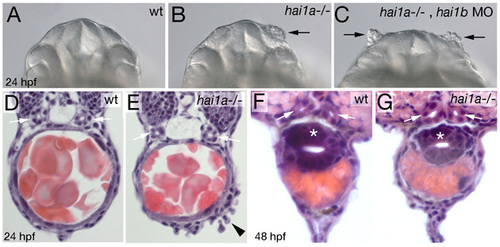
Loss of Hai1 activity affects morphology of the olfactory epithelium, but not the morphology of the pronephric ducts and the gut. (A-C) Frontal views on head of live embryos at 24 hpf. Black arrows point to dissociating olfactory epithelium in hai1a mutant (with low penetrance and expressivity; B) and hai1a mutant injected with hai1b MO (with high penetrance and expressivity; C). (D-G) Eosin/hematoxylene stainings of cross-sections through posterior trunk region of hai1a mutants (E,G) and wild-type siblings (D,F) at 24 hpf (D,E) or 48 hpf (F,G). Pronephric ducts are indicated with white arrows, the gut in (F,G) with white asterisks. Black arrowhead in (E) points to dissociating epidermis on yolk extension. PHENOTYPE:
|
|
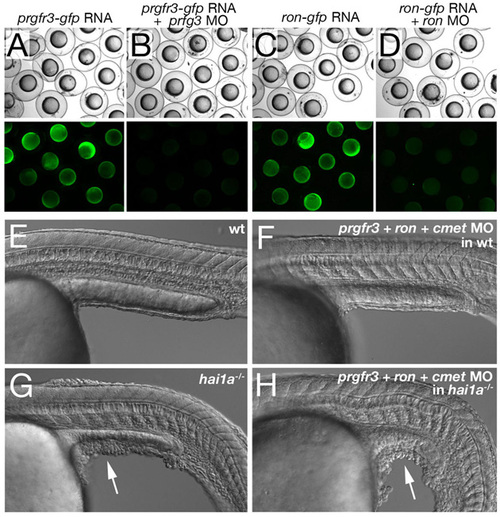
Concomitant inactivation of cMet and its related receptors Prgfr3 and Ron is insufficient to rescue the epidermal defects of hai1a mutants. (A-D) prgfr3 and ron MOs efficiently block the translation of their respective mRNAs encoding Prgfr3-GFP or Ron-GFP fusion proteins. Upper panels show bright-field images, lower panel GFP fluorescence of embryos at the 80% epiboly stage, after injection of prgfr3-gfp mRNA (A,B) or ron-gfp mRNA (C,D), and with (B,D) or without (A,C) the respective MOs. Co-injection of MOs leads to complete suppression of GFP fluorescence. (E-F) Lateral Nomarski images of 24 hpf un-injected wild-type embryo (E) and wild-type embryos co-injected with cmet MO, prgfr3 MO and ron MO (each at 0.16 mM) (F), revealing that all three receptors are dispensable for normal skin development. (G,H) Lateral Nomarski images of 24 hpf un-injected hai1a mutant (G) and hai1a mutant co-injected with cmet MO, prgfr3 MO and ron MO (each at 0.16 mM), revealing that Hai1 does not act by inactivating cMet, Prgfr3 and Ron. White arrows point to dissociating epidermis on yolk extension. |