Fig. S1
- ID
- ZDB-FIG-161122-7
- Publication
- Bhattarai et al., 2016 - IL4/STAT6 Signaling Activates Neural Stem Cell Proliferation and Neurogenesis upon Amyloid-?42 Aggregation in Adult Zebrafish Brain
- Other Figures
- All Figure Page
- Back to All Figure Page
|
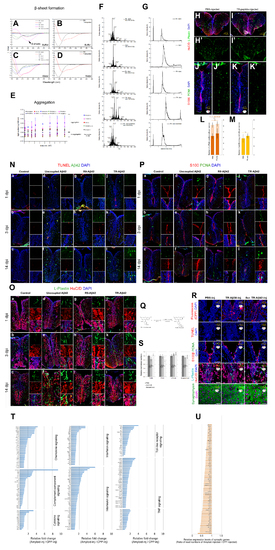
Chemical properties and effects of various amyloid beta peptides and expression changes in inflammation-related and synaptic genes. Related to Figure 1, Figure 2. (A) Circular Dichroism (CD) spectra for uncoupled A?42, TR-A?42, R9-A?42 and scrambled A?42 in buffer (PBS). A?42 peptides show ?-sheet formation in buffer. (B) CD for CPPs alone in buffer. They do not show beta structures. (C) Circular Dichroism (CD) spectra for uncoupled A?42, TR-A?42, R9-A?42 and scrambled A?42 in water. A?42 peptides do not show ?-sheet formation in water. (D) CD for CPPs in water. They do not show beta structures. (E) Light scattering analyses to detect aggregation for R9-A?42, TR-A?42, A?42 scrambled and R9-A?38 peptides in water and buffer. A?42 peptides aggregate in buffer significantly more than A?42 scrambled and A?38. No peptide aggregates in water. (F) Mass by charge ratio of the native and modified and amyloid beta peptides. (G) Liquid Chromatography - Mass Spectrometry of HPLC-purified native and modified and amyloid beta peptides. (H) Immunostaining for HuC/D and L-Plastin on PBS-injected brains. (H?) Green channel alone. (I) Immunostaining for HuC/D and L-Plastin on TR peptide-injected brains. (I?) Green channel alone. (J) Immunostaining for S100beta and PCNA on PBS-injected brains. (J?) Green channel alone. (K) Immunostaining for S100b and PCNA on TR peptide-injected brains. (K?) Green channel alone. (L) Quantification graph for L-Plastin cells. TR peptide does not alter the number and activation state of the microglia. (M) Quantification graph for radila glial cell proliferation. TR peptide does not alter the proliferation state of the adult zebrafish brain. (N) TUNEL and A?42 staining for control (a-c), uncoupled A?42 (d-f), R9-A?42 (g-i), TR-A?42 (j-l) at 1-, 3- and 14 days post injection (dpi). small insets on the right of every panel are individual fluorescence channels and the composite from a close-up region. (O) L-Plastin and HuC/D staining for control (a-c), uncoupled A?42 (d-f), R9-A?42 (g-i), TR-A?42 (j-l) at 1-, 3- and 14 days post injection (dpi). small insets on the right of every panel are individual fluorescence channels and the composite from a close-up region. (P) S100 and PCNA staining for control (a-c), uncoupled A?42 (d-f), R9-A?42 (g-i), TR-A?42 (j-l) at 1-, 3- and 14 days post injection (dpi). small insets on the right of every panel are individual fluorescence channels and the composite from a close-up region. (Q) Coupling reaction for attaching fluorescein to peptides. (R) Fluorescein, TUNEL, S100? and PCNA, L-Plastin and HuC/D, Synptophysin immunostainings for PBSinjected, TR-A?38-injected and TR-A?42-scrambled peptide injected brains. (S) Quantification graphs for R. (T) Relative fold changes of inflammation-related genes. (U) Relative fold changes of synapse-related genes. Scale bars: 50 ?m. Data are represented as mean ± SD. |