- Title
-
Axon demyelination and degeneration in a zebrafish spastizin model of hereditary spastic paraplegia
- Authors
- Garg, V., André, S., Heyer, L., Kracht, G., Ruhwedel, T., Scholz, P., Ischebeck, T., Werner, H.B., Dullin, C., Engelmann, J., Möbius, W., Göpfert, M.C., Dosch, R., Geurten, B.R.H.
- Source
- Full text @ Open Biol.
|
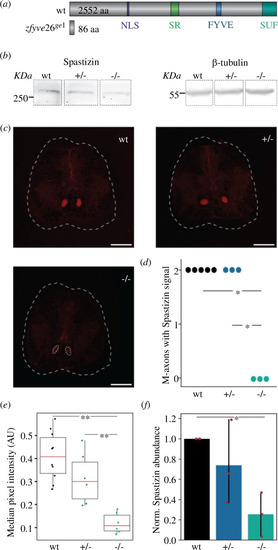
Effects of CRISPR-Cas9 mediated gene editing on Spastizin abundance. ( |
|
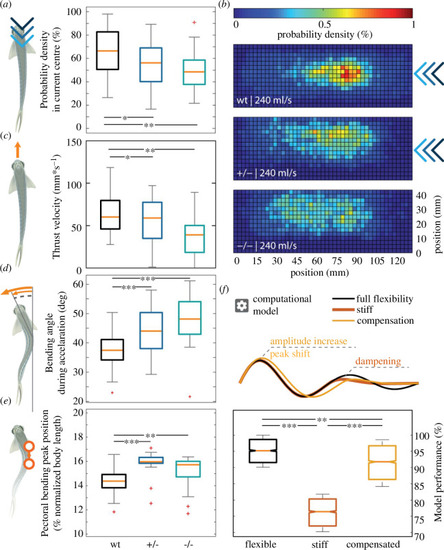
Locomotion defects observed in |
|
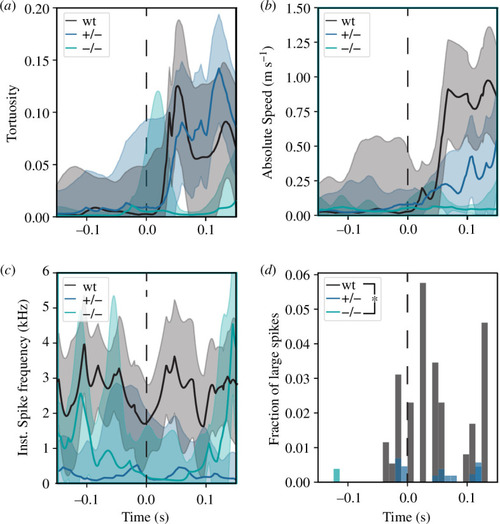
Lack of Spastizin affects the function of M-cells. ( |
|
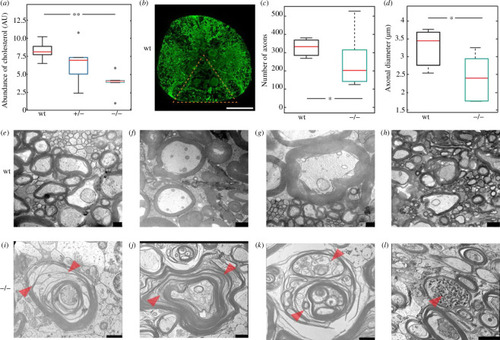
Degeneration and demyelination of the spinal cord motor neuron axons of the |