- Title
-
Biallelic BORCS8 variants cause an infantile-onset neurodegenerative disorder with altered lysosome dynamics
- Authors
- De Pace, R., Maroofian, R., Paimboeuf, A., Zamani, M., Zaki, M.S., Sadeghian, S., Azizimalamiri, R., Galehdari, H., Zeighami, J., Williamson, C.D., Fleming, E., Zhou, D., Gannon, J.L., Thiffault, I., Roze, E., Suri, M., Zifarelli, G., Bauer, P., Houlden, H., Severino, M., Patten, S.A., Farrow, E., Bonifacino, J.S.
- Source
- Full text @ Brain
|
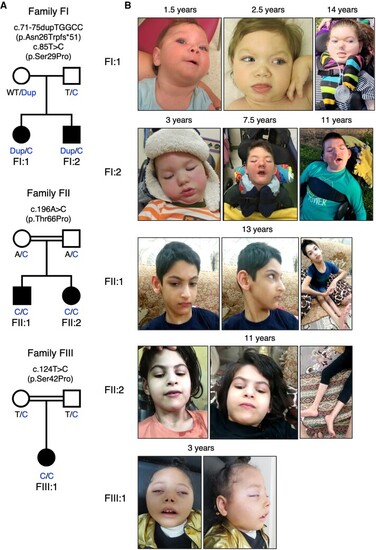
Family pedigrees and photographs of the patients. (A) Family pedigrees and genotypes. BORCS8 variants are indicated, with Dup corresponding to the c.71-75dupTGGCC (p.Asn26Trpfs*51) variant, and T/C and A/C to the T>C and A>C single-base substitution variants, respectively. (B) Photographs of the patients at the indicated ages. |
|
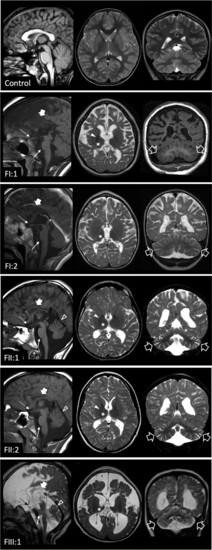
Neuroimaging findings. Brain MRI studies of a control subject performed at 9 years of age for comparison, and of the patients performed at 9 years (Patient FI:1), 7.7 years (Patient FI:2), 13 years Patient FII:1), 12 years (Patient FII:2) and 3 years of age (Patient FIII:1). In the patients, sagittal T1- or T2-weighted (left), axial T2-weighted (middle) and coronal T1 or T2-weighted images (right) reveal mild-to-severe cerebral atrophy with reduced white matter volume and enlarged subarachnoid spaces in all cases. There is diffuse T2 hyperintensity of the cerebral white matter in all subjects, with relative sparing of the internal capsules and subcortical U-fibres in Patients FII:1 and FII:2, in keeping with hypomyelination. Focal signal alterations are associated at the level of the fronto-parietal white matter. The corpus callosum is thin in all patients (thick arrows). The thalami are very small and slightly T2 hypointense in all cases (asterisks). The globi pallidi are small and darker on T2-weighted images in Patients FI:1, FII:1 and FII:2 (full arrowheads). There is mild pontine hypoplasia (thin arrows) and marked optic nerve and chiasm atrophy (thin dashed arrows) in all subjects. Mild-to-moderate cerebellar atrophy with prevalent enlargement of the hemispheric subarachnoid spaces is present in all subjects (empty arrows), while involvement of the superior vermis is visible only in Patients FII:1, FII:2 and FIII:1 (empty arrowheads). |
|
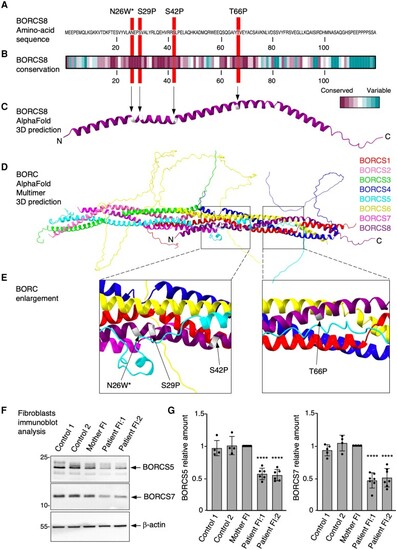
Characteristics of wild-type and variant BORCS8 alleles. (A) Amino acid sequence of wild-type BORCS8 indicating the positions of the variants. (B) Amino acid conservation of BORCS8 from different species calculated on the ConSeq server using default search values. (C) Structure of BORCS8 extracted from the whole BORC complex predicted by AlphaFold Multimer. (D) Structure of the BORC complex predicted by AlphaFold Multimer. (E) Close-up view of the positions of the variants. (F) Skin fibroblast cultures from two unrelated controls [a healthy individual (Control 1, 85E0344) and an SPG50 patient (Control 2, 87RD39)],, the unaffected Family FI mother and her affected children were analysed by SDS-PAGE and immunoblotting for the endogenous BORCS5 and BORCS7 subunits of BORC. ?-Actin was used as loading control. The positions of molecular mass markers (in kDa) are indicated on the left. (G) Quantification of endogenous BORCS5 and BORCS7 levels from at least four independent experiments such as that shown in F. Values are the mean ± standard deviation (SD) from the number of data-points shown on the figure. Statistical significance was calculated by one-way ANOVA followed by multiple comparisons using Dunnett?s test. ****P < 0.0001. |
|
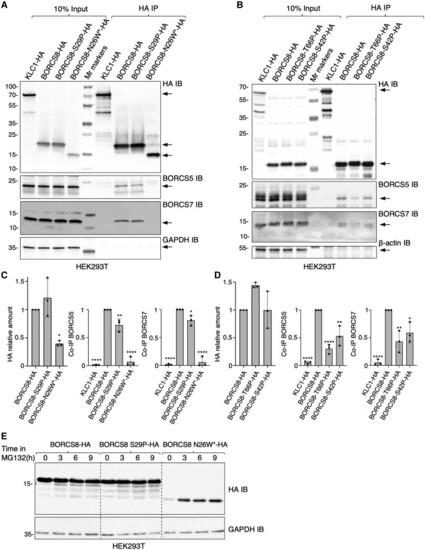
BORCS8 patient variants reduce the levels and/or assembly of BORC subunits. (A and B) HEK293T cells were transfected with plasmids encoding the indicated HA-tagged constructs and subjected to immunoprecipitation with antibody to the HA epitope. Cell extracts (10%) and immunoprecipitates (HA IP) were analysed by SDS-PAGE and immunoblotting (IB) for the HA epitope, endogenous BORCS5 and BORCS7, GAPDH (control) or ?-actin (control). KLC1-HA was used as a non-specific immunoprecipitation control. The positions of molecular mass markers (in kDa) are indicated on the left. Arrows indicate the positions of the specific proteins. (C and D) Quantification from three independent experiments such as those shown in A and B. Values are the mean ± standard deviation (SD). Statistical significance was calculated by one-way ANOVA followed by multiple comparisons using Dunnett?s test. *P < 0.05; **P < 0.01; ****P < 0.0001. (E) HEK293T cells transfected with HA-tagged BORCS8 constructs were treated with 40 ?M of the proteasomal inhibitor MG132 for the indicated times, lysed and immunoblotted for the HA epitope and GAPDH (loading control). The positions of molecular mass markers (in kDa) are indicated on the left. |
|
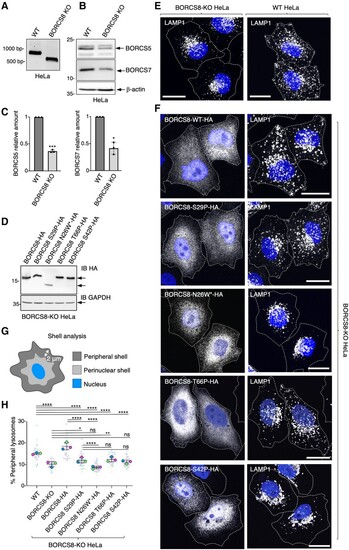
BORCS8 patient variants impair the lysosome-dispersal function of BORC. (A) Agarose gel electrophoresis and GelRed® staining of genomic BORCS8 PCR products yields 745-bp fragment for wild-type (WT) and 513-bp fragment for BORCS8-KO HeLa cells. (B) SDS-PAGE and immunoblot analysis of wild-type and BORCS8-KO HeLa cells using antibodies to endogenous BORCS5 and BORCS7. ?-Actin was used as a control. The positions of molecular mass markers (in kDa) are indicated on the left. (C) Quantification of BORCS5 and BORCS7 levels from three independent experiments such as that shown in B. Values are the mean ± standard deviation (SD). Statistical significance was calculated by Student?s t-test. *P < 0.05; ***P < 0.001. (D) BORCS8-KO HeLa cells were transiently transfected with the indicated HA-tagged BORCS8 constructs and cell extracts were immunoblotted (IB) for the HA epitope and GAPDH (loading control). The positions of molecular mass markers (in kDa) are indicated on the left. The positions of specific proteins are indicated by arrows. (E) Wild-type and BORCS8-KO HeLa cells were fixed, permeabilized and immunostained for the endogenous lysosomal protein LAMP1. Nuclei were labelled with DAPI (blue). Cell edges were outlined by staining of actin with fluorescent phalloidin (not shown) and indicated by dashed lines. Scale bars = 20 ?m. (F) BORCS8-KO cells were transiently transfected with the indicated HA-tagged constructs and immunostained for the HA epitope and endogenous LAMP1 as described for E. (G) Schematic representation of shell analysis. (H) Quantification by shell analysis of peripheral LAMP1 signal in HeLa cells from three experiments such as those shown in E and F. Data are presented as SuperPlots showing the individual data-points, the mean from each experiment, and the mean ± SD of the means. Statistical significance was calculated by one-way ANOVA followed by multiple comparisons using Tukey?s test. *P < 0.05; **P < 0.01; ****P < 0.0001; ns = not significant. |
|
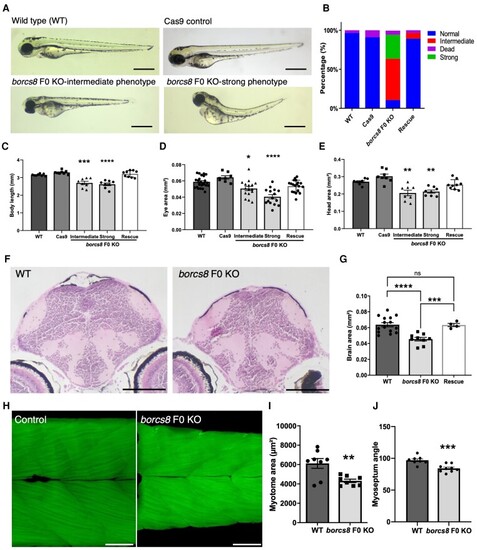
Zebrafish borcs8 F0 knockout larvae exhibit developmental defects. (A) Morphology of zebrafish wild-type (WT), Cas9 control and borcs8 F0 knockout (KO) larvae at 3 days post-fertilization (dpf). Scale bars = 1 mm. (B) Frequency of phenotypes observed for wild-type, Cas9 control, borcs8 F0 KO and human BORCS8 mRNA rescue (Rescue) larvae (N = 4, n = 32?72). (C?E) Body length (C), head size (D) and eye size (E) of wild-type, Cas9 control, borcs8 F0 KO and Rescue larvae at 3 dpf (N = 3, n = 8?9 for head size and body length, n = 16?18 for eye size). (F) Haematoxylin and eosin staining of paraffin-embedded brain sections from the midbrain of 5 dpf wild-type and borcs8 F0 KO larvae. Scale bar = 100 ?m. (G) Quantification of brain size of borcs8 F0 KO larvae (N = 9) relative to wild-type (N = 15) and Rescue (N = 4) at 5 dpf. (H) Phalloidin staining of muscles in borcs8 F0 KO and wild-type larvae at 3 dpf. (I and J) Comparisons of dorsal or ventral myotome area (I) and myoseptum angle (J) between wild-type (N = 3, n = 8) and borcs8 F0 KO larvae (N = 3, n = 8?9). All data are represented as the mean ± standard error of the mean (SEM). Statistical significance was calculated by one-way ANOVA followed by Tukey?s multiple comparisons tests, or Student?s t-test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. N = replicates; n = sample size; ns = not significant. PHENOTYPE:
|
|
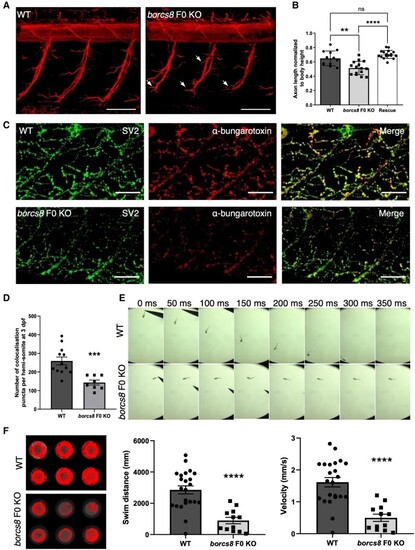
Neuromuscular defects and impaired motility in borcs8 F0 knockout zebrafish larvae. (A) Primary motor axons in wild-type (WT) and borcs8 F0 knockout (KO) larvae at 3 days post-fertilization (dpf). Scale bars = 50 ?m. Defects in axon branching are indicated by white arrows. (B) Quantification shows marked reduction in the axon length of motor neurons in borcs8 F0 KO (N = 3, n = 15) compared to WT (N = 3, n = 12) and Rescue larvae (N = 3, n = 18). (C) Co-immunostaining of zebrafish neuromuscular junctions (NMJs) with presynaptic (SV2; green) and postsynaptic (?-bungarotoxin; red) markers in 3 dpf zebrafish. Scale bars = 50 ?m. (D) Quantification of co-localizing presynaptic and postsynaptic markers per hemisomite, normalized to the number of presynaptic puncta, showed a significant reduction in the number of puncta in borcs8 F0 KO larvae (N = 3?4, n = 8?12). (E) Representative snapshots of touch escape responses (N = 6) over 350 ms. Zebrafish borcs8 F0 KO larvae have very little to no escape response at 2 dpf. (F) Representative swimming tracks of wild-type control and borcs8 F0 KO fish at 5 dpf. The borcs8 F0 KO larvae (N = 3, n = 32) displayed impaired swim distance and velocity compared to controls (N = 3, n = 24). All data are represented as the mean ± standard error of the mean (SEM). Statistical significance was calculated by Student?s t-test, or one-way ANOVA followed by Tukey?s multiple comparisons tests. **P < 0.01; ***P < 0.001; ****P < 0.0001. N = replicates; n = sample size; ns = not significant. EXPRESSION / LABELING:
|