- Title
-
Intracellular Calcium Mobilization Is Required for Sonic Hedgehog Signaling
- Authors
- Klatt Shaw, D., Gunther, D., Jurynec, M.J., Chagovetz, A.A., Ritchie, E., Grunwald, D.J.
- Source
- Full text @ Dev. Cell
|
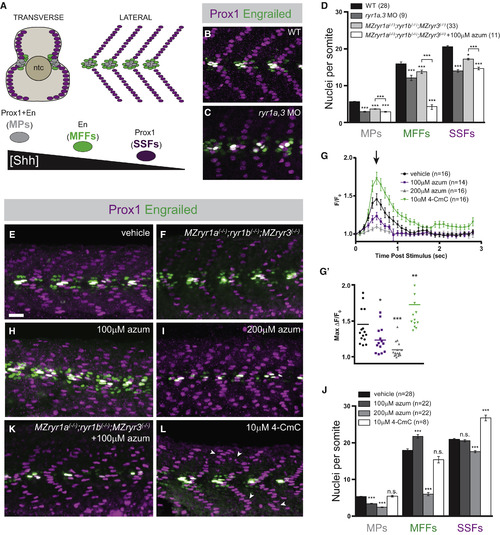
Formation of Shh-Dependent Muscle Requires RyR Function (A) Schematic illustration of the nuclei of Shh-dependent muscle cells in the somites of 26 hpf zebrafish embryos, presented relative to the notochord (ntc) in transverse section and in a superficial lateral view (anterior to left). Nuclei of different muscle types are color-coded: slow muscle pioneer cells ([MPs], gray), medial fast fibers ([MFFs], green), and superficial slow fibers ([SSFs], magenta). (B, C, E, F, H, I, K, and L) Shh-dependent muscle fiber nuclei, marked by Prox1 (magenta) and En (green) expression, were visualized by immunostaining 26 hpf embryos: (B) wild-type (WT), (C) ryr1a and ryr3 MO-injected (ryr1a,3 MO), (E) vehicle-treated (0.5% DMSO) WT, (F) MZryr1a(?/?);ryr1b(?/?);MZryr3(?/?) mutant, (H and I) azumolene-treated WT, (K) azumolene-treated MZryr1a(?/?);ryr1b(?/?);MZryr3(?/?) mutant, and (L) 4-CmC-treated WT (supernumerary Prox1+ SSF nuclei are indicated with arrowheads). All nuclei co-expressing En and Prox1 were considered MPs. Scale bar, 25 ?m. (D and J) Quantification of distinct muscle cell types per somite. Data are represented as means ± SEM. As numbers of nuclei in WT and DMSO-treated embryos did not differ significantly, comparisons were made with control vehicle-treated embryos unless otherwise indicated. ?p < 0.01; ???p < 0.0001; n.s., not significant. (G) Traces of GCaMP fluorescence in electrically stimulated muscle of 24 hpf Tg(act2b:GCaMP6f) embryos incubated from 6 to 24 hpf in indicated solutions. Data are represented as means ± SEM. The maximum change in fluorescence from baseline, arrow in (G) is shown for each recorded embryo in (G?) with horizontal lines representing means. ?p < 0.01, ??p < 0.001, ???p < 0.0001. See also Figure S2. |
|
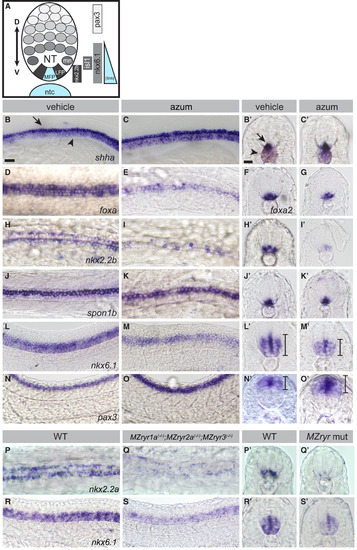
RyR Function Is Required for Shh-Dependent Patterning of the Neural Tube (A) Schematic of Shh-dependent neural tube (NT) patterning. The medial floorplate (MFP), lateral floorplate (LFP), and neighboring motoneurons (mn) are indicated. The notochord (ntc) and MFP are sources of Shh, designated in blue. Approximate gene expression domains of nkx2.2b, isl1, nkx6.1, and pax3 are indicated. (B?O) Whole-mount in situ hybridization to detect neural tube markers in vehicle-treated (0.5% DMSO) and 200 ?M azumolene-treated embryos. (B and C) shha-expression in the notochord (arrowhead) and MFP (arrow) is similar in control and treated embryos. (D?O) Gene expression in the neural tube is shifted upon azumolene treatment in a manner consistent with reduced Shh signaling. (D?G) Whereas foxa and foxa2 are expressed in both the MFP and LFP in control embryos, they are expressed in fewer FP cells in azumolene-treated embryos. (H?K) Expression of nkx2.2b marking the LFP is reduced, but spon1b expression marking the MFP appears unchanged in azumolene-treated embryos. (L?O) The ventral domain of the neural tube, marked by nkx6.1, is reduced, while the dorsal domain of the neural tube, marked by pax3, is expanded in azumolene-treated embryos. (P?S) Expression of nkx2.2a in the LFP and nkx6.1 in the ventral domain of the neural tube is diminished in MZryr1a(?/?);MZryr2a(?/?);MZryr3(?/?) compared with WT. (B, C, L?O, R, and S) Lateral views of flat mounted embryos. (D, E, H?K, P, and Q) Dorsal views of flat-mounted embryos. Prime letters, (F), and (G) are transverse cross-sections. Scale bars, 25 ?m. Interval bars indicate dorsoventral extents of gene expression domains. See also Figure S3. |
|
RyR Function Is Required for Shh-Dependent Gli-Mediated Gene Expression (A?H) Dorsal view images of live Tg(8xGli:mCherry-NLS-Odc1) embryos at 12 hpf (A, C, E, and G) or 24 hpf (B, D, F, and H) that had been treated with vehicle (0.5% DMSO), cyclopamine, azumolene, or ryanodine. At 12 hpf, the position of the notochord (ntc) just ventral to the FP is outlined by dashed lines. (A) In control 12 hpf embryos, mCherry is expressed in nuclei of cells responding to Shh, including adaxial cells (arrowhead) and FP cells (arrow). (B) At 24 hpf, mCherry is expressed in nuclei of slow muscle cells (arrowhead) and cells in the ventral neural tube (arrow). (C?H) mCherry expression is reduced in embryos treated with each drug. (I) Tail-transected Tg(8xGli:mCherry-NLS-Odc1) embryos were soaked in vehicle or 4-CmC from 16 to 18 hpf, fixed, and imaged. (J?L) Potentiation of RyR channel activity with 4-CmC treatment results in increased numbers of presumptive slow muscle nuclei that express the mCherry reporter (J and K are lateral views, arrowhead indicates nuclei of slow muscle cells and arrow indicates cells in the ventral neural tube). (L) Quantification of mCherry+ nuclei per somite in 4-CmC-treated embryos. Each point represents a single somite and the horizontal line represents the mean. ??p < 0.001. (M?Q) RyR activity affects endogenous ptch2 expression as detected by whole-mount in situ hybridization in 24 hpf embryos. (M?Q) Lateral views reveal expression in somites and (M??Q?) transverse sections reveal expression in slow muscle cells surrounding the notochord and in the ventral neural tube. Compared with WT embryos, ptch2 expression is diminished in azumolene-treated and MZryr1a(?/?);MZryr2a(?/?);MZryr3(?/?) mutant embryos, and it is enhanced in 4-CmC-treated embryos. Scale bars, 25 ?m. EXPRESSION / LABELING:
PHENOTYPE:
|
|
RyR Function Is Required for Development of Shh-Dependent Neural Crest-Derived Neurons of the DRG and the ENS (A?L) Lateral views of live 72 hpf Tg(isl2b:GFP) embryos with GFP-labeled Rohon-Beard neurons (arrow) and DRGs (arrowhead). Embryos were treated between 24 and 48 hpf with cyclopamine (cyc), azumolene (azum), ryanodine (ryan), N-acetyl cysteine (NAC), aldrithiol, thapsigargin (thaps), or tricaine at the indicated concentrations, or injected at the one-cell stage with ryr1a and ryr3 MOs. Arrowheads in (C and G) indicate the presence of small, faint DRGs. (M) Quantification of embryos treated as in (A?L). DRGs present in somites 11?15 were counted. For each condition, the number of embryos analyzed is indicated. Comparisons are to control vehicle-treated embryos unless otherwise indicated. Data are represented as means ± SEM. ?p < 0.01; ???p < 0.0001; n.s., not significant. (N?Q) Lateral views of live 78 hpf Tg(phox2bb:GFP) embryos with the ENS (arrow) labeled by GFP in control, cyclopamine-treated, ryr1a,3 morphant, or ryanodine-treated embryos. Black melanophores (pigment) are visible in each condition. Scale bar, 25 ?m in (A). See also Figure S4. EXPRESSION / LABELING:
PHENOTYPE:
|
|
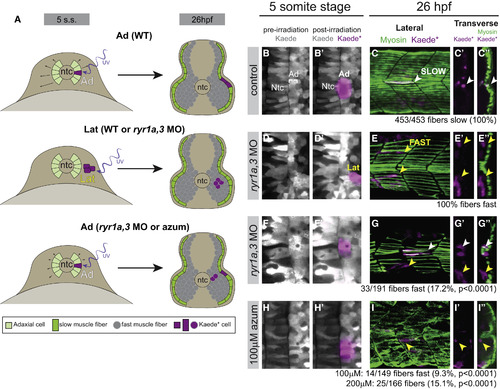
Adaxial Precursors Transfate to Produce Shh-Independent Fast Muscle Derivatives in Embryos with Diminished RyR Function (A) Schematic representation of experimental setup and summary of results, with embryos shown in transverse views. At the five-somite stage Kaede was photoconverted to Kaede? in small groups of adaxial (Ad) slow muscle precursors or lateral (Lat) fast muscle precursors. At 26 hpf (14 hr later), embryos were fixed and stained for F59 myosin expression. (B, D, F, and H) Labeling of muscle precursor cells for lineage analysis. Clusters of slow muscle precursor adaxial (B and B?, F and F?, and H and H?) or fast muscle precursor lateral (D and D?) cells were labeled by photoconversion (magenta) at the five-somite stage. At 26 hpf, the positions of Kaede?-labeled (magenta) cells in the somites were determined relative to the superficial slow muscle, detected with the F59 antibody (myosin, green). (C?C?) Kaede?-labeled adaxial cells always gave rise to F59+ descendants in the parallel array of superficial slow muscle cells (white arrowheads) in control embryos. (E?E?) Kaede?-labeled lateral somite precursors always became deep fast muscle regardless of ryr gene expression (yellow arrowheads). (G?G? and I?I?) In ryr1a,3 morphant or azumolene-treated embryos, adaxial cells gave rise to both slow (white arrowheads) and fast (yellow arrowheads) muscle fibers. |
|
RyR Function Is Required in the Shh Ligand-Receiving Cell (A) Design of mosaic analysis experiments. Spatially separated NC progenitors and dorsal organizer (shield) are indicated. Formation of donor-derived Rohon-Beard (RB) and DRG neurons in mosaic embryos was determined at 78 hpf. (B?D) Examples of chimeric embryos indicating the differentiation of donor-labeled Tg(isl2b:GFP) tissue in unlabeled hosts. (B and C) Donor WT cells gave rise to both DRGs (arrows) and RBs (arrowheads) when they developed in either WT or ryr1a,3 morphant hosts. (D) ryr1a,3 morphant cells failed to produce DRGs in WT hosts. (E) The numbers of GFP-labeled RBs and DRGs present in each mosaic embryo is plotted. Lines representing best-fit analyses indicate that WT host donor cells gave rise to DRGs and RBs in a 2:3 ratio (black and magenta lines with slopes of 0.64 and 0.69, respectively). In contrast, donor cells from ryr1a,3 morphant embryos gave rise to RBs, but not DRGs (green line with a slope of 0.06). (F?M) Prox1 and En expression at 26 hpf in (F and G) shha RNA-injected, (H and I) MZptch2(?/?), (J and K) SmoM2 RNA-injected, and (L and M) dnPKA RNA-injected embryos. Azumolene treatment attenuated overproduction of Shh-dependent muscle cell types activated by Shh overexpression, loss of the Patched2 receptor, or overexpression of SmoM2. In contrast, azumolene failed to alter the development of muscle cells in embryos expressing dnPKA. Scale bar, 25 ?m in (F). See also Figures S5 and S6. |
|
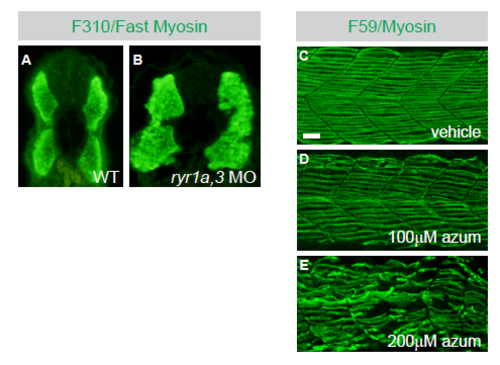
Formation of Shh-dependent muscle, but not Shh-independent fast muscle, requires RyR function. Related to Figure 1. (A and B) Immunohistochemistry with the F310 antibody to detect fast muscle myosin in Tu control and ryr1a,3 MO-treated embryos at 30hpf. Images are transverse cross sections. (C-E) Slow muscle fiber numbers were reduced following treatment with azumolene. Slow muscle fibers were detected at 26hpf with F59 antibody recognizing myosin in (C) vehicle control, (D) 100?M azumolene, and (E) 200?M azumolene-treated embryos. Scale bar indicates 25?m. |
|
RyR function is necessary for the Shh-dependent patterning of the neural tube. Related to Figure 2. (A and B) Development of motoneurons, but not Rohon Beard sensory neurons of the neural tube is diminished by azumolene treatment. Isl1 expression marks sensory Rohon Beard (arrow) and motor neurons (arrowhead) in 24hpf embryos. Rohon Beard neurons develop independently of Shh signaling. Scale bar indicates 25?m. (C and D) Gene expression domains in the neural tube are shifted along the dorsoventral axis in azumolene-treated or MZryr1a(-/-);MZryr2a(-/-);MZryr3(-/-) embryos. Quantification of the dorsoventral extent of the nkx6.1 and pax3 expression domains. Each point represents a single embryo and the line indicates the mean. *p<0.01; **p<0.001; ***p<0.0001. |
|
Azumolene treatment inhibits calcium mobilization and fin development, but not trunk neural crest migration. Related to Figure 4. (A) Traces of GCaMP fluorescence in electrically stimulated muscle of 1 day Tg(act2b:GCaMP6f) embryos. Caudal tips of tails were transected at 1 day and embryos were incubated in indicated solutions for 30 minutes at which time muscle was re-stimulated. Before cut (purple) indicates embryos prior to tail transection and drug treatment. Data are represented as mean ±SEM. The maximum change in fluorescence from baseline (arrow in A) is shown for each recorded embryo in (A?) with horizontal lines representing means. *p<0.01; ***p<0.0001; n.s., not significant. (B and C) Streams of migrating neural crest in the trunk, revealed by whole-mount in situ hybridization to detect crestin RNA expression, appear normal in ryr1a,3 morphants as compared to Tu controls. (D-G) RyR function is necessary for the Shh-dependent formation of pectoral fins. (D) Schematic dorsal view of 72hpf zebrafish embryo with region of interest outlined in box. Proximal/distal axis is indicated by blue arrow. (E-G) Images of fin buds in 72hpf embryos treated from 24 to 48hpf with indicated pharmacological agents. |
|
Azumolene reduces ectopic muscle allocation and DRG formation caused by overactivation of the Shh signaling pathway. Related to Figure 6. (A) Quantification of Shh-dependent muscle cell types (as determined by transcription factor expression in nuclei) in embryos with experimentally activated Shh signaling. Data are derived from experiments illustrated in Figures 6F-6M. For each condition, the number of embryos analyzed is indicated. Fold change in the number of nuclei was calculated relative to 0.5% DMSO-treated controls. Data are represented as mean ±SEM. *p<0.01; ***p<0.0001; n.s., not significant. (B-D) Mutants that fail to express functional Patched2 receptor have an overproduction of DRGs, which is reduced by treatment with azumolene. Pan-neuronal staining with anti-HuC was used to mark DRGs (white arrowheads). (C) MZptch2(-/-) mutants have an increased number of DRGs, some of which form in ectopic locales (yellow arrowheads). (D) DRG formation in MZptch2(-/-) embryos is eliminated by azumolene treatment. (E) Quantification of Figures S5B-S5D. Each point represents a single embryo and the line indicates the mean. *p<0.01; ***p<0.0001. |
|
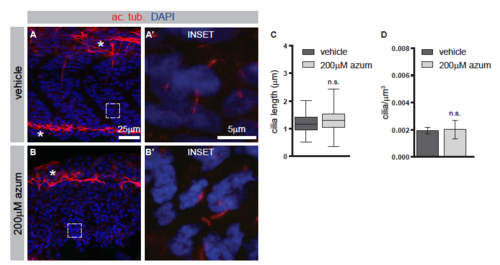
Azumolene does not affect the formation of primary cilia. Related to Figure 6. (A and B) Immunohistochemistry to detect acetylated tubulin (ac. tub.), which marks primary cilia (red), and DAPI-staining to indicate nuclei (blue). Neural axons are also labeled by ac. tub. and are indicated by asterisks. Higher magnification views in A? and B? are indicated by white boxes. (C and D) Quantification of the lengths of the ciliary axoneme or the number of cilia per micron cubed. In C, boxes indicate 50th percentile, whiskers indicate the minimum and maximum values, and median is indicated by the line. In D, data are represented as mean ±SEM. n.s., not significant. |
Reprinted from Developmental Cell, 45(4), Klatt Shaw, D., Gunther, D., Jurynec, M.J., Chagovetz, A.A., Ritchie, E., Grunwald, D.J., Intracellular Calcium Mobilization Is Required for Sonic Hedgehog Signaling, 512-525.e5, Copyright (2018) with permission from Elsevier. Full text @ Dev. Cell