- Title
-
DeltaC and DeltaD interact as Notch ligands in the zebrafish segmentation clock
- Authors
- Wright, G.J., Giudicelli, F., Soza-Ried, C., Hanisch, A., Ariza-McNaughton, L., and Lewis, J.
- Source
- Full text @ Development
|
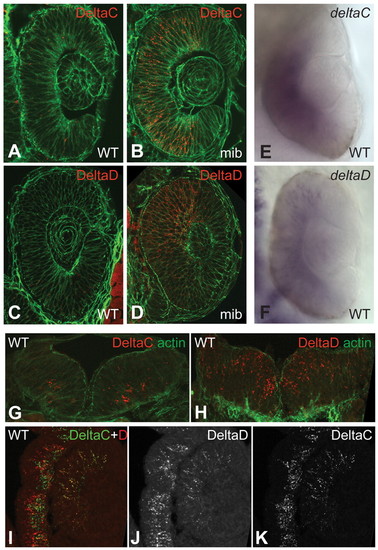
Expression of DeltaC and DeltaD in retina and brain. (A-D) Sections of wild-type (WT) and mib mutant zebrafish retina at 24 hpf, immunostained with zdc2 and zdd2. (E,F) In situ hybridisation (ISH) of wild-type specimens (wholemounts) with probes detecting deltaC and deltaD mRNA. (G,H) Sections of hindbrain at 24 hpf, immunostained for DeltaC and DeltaD. (I-K) Section of retina and adjacent brain tissue in a 48 hpf embryo, doubly immunostained for DeltaC and DeltaD. In the retina the two proteins largely colocalise, but in the brain they are in distinct sets of cells. A-D and I-K are single confocal optical sections of 15 Ám cryosections; G,H are projections of a small z-stack. A-D and G,H are counterstained for actin with fluorescent phalloidin (green). |
|
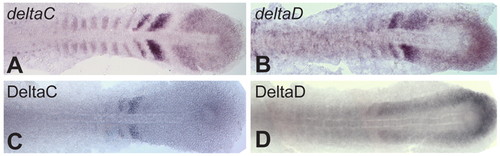
ISH and antibody staining patterns compared in flat-mounted ~10-somite stage zebrafish embryos. (A,B) ISH for deltaC and deltaD. (C,D) Immunohistochemical staining of DeltaC with zdc2 and of DeltaD with zdd2. |
|
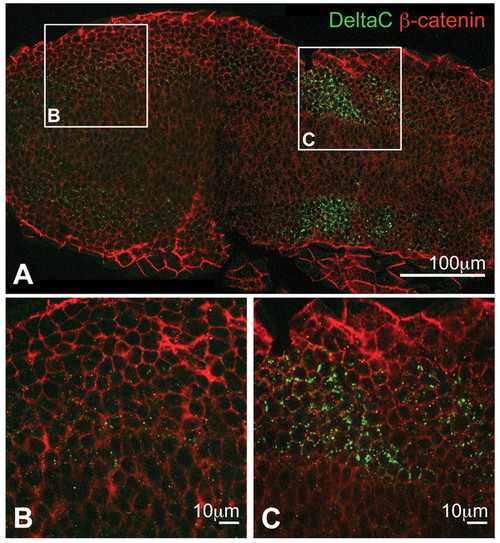
Optical sections of PSM immunostained for DeltaC and β-catenin in a flat-mounted, 10-somite stage wild-type zebrafish embryo. (A) Low-magnification overview. DeltaC, green; β-catenin, red. (B,C) Higher magnifications of the boxed regions in A showing posterior (B) and anterior (C) presomitic mesoderm (PSM). DeltaC staining is always observed as intracellular puncta, with very faint expression in the posterior but strong upregulation in the anterior PSM. |
|
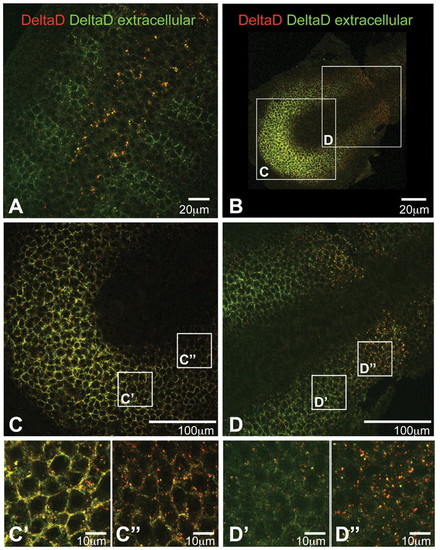
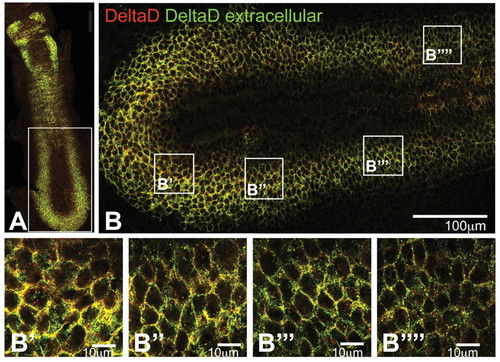
Optical sections of flat-mounted embryos stained to determine the ratio of extracellular to intracellular DeltaD. zdd2 staining before permeabilisation is in green and that after permeabilisation is in red. A low red:green ratio indicates extracellular staining, whereas a high red:green ratio indicates intracellular. (A) Trunk region of a 16 hpf wild-type zebrafish embryo: DeltaD is mainly intracellular and granular in presumptive neuroblasts of the neural tube, but is mainly at the cell surface in the anterior part of each somite. (B-D′′) PSM of a 10-somite stage wild-type embryo. In the posterior PSM, DeltaD is mainly located at the cell membrane (yellow cell outlines in C-C′′); in the anterior PSM DeltaD is predominantly intracellular (red dots in D-D′′). |
|
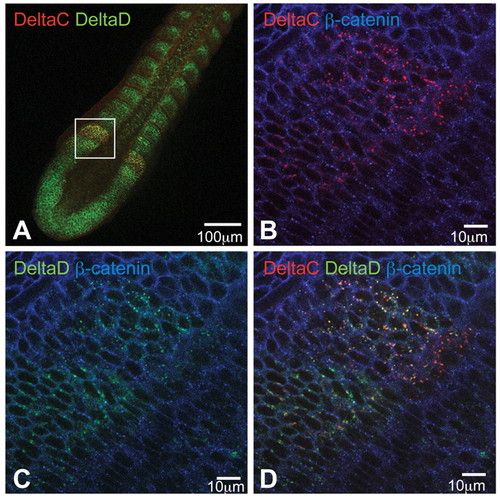
DeltaC and DeltaD are colocalised in puncta in the anterior PSM. (A-D) Optical sections of a flat-mounted 14-somite stage wild-type zebrafish embryo immunostained for DeltaC (red), DeltaD (green) and β-catenin (blue). DeltaC and DeltaD are upregulated in the same region within the anterior PSM and colocalise within the same intracellular granules. |
|
The subcellular localisation of DeltaD in the PSM of a mib mutant zebrafish embryo. (A-B′′′′) Optical sections of a flat-mount immunostained with zdd2 before (green) and after (red) permeabilisation, as in Fig. 6, to distinguish between intracellular and extracellular DeltaD. The caudorostral gradients in the quantity of DeltaD per cell and in its degree of internalisation, as evident in wild type (see Fig. 6), are lost in the mib mutant, producing a uniform distribution of DeltaD that is at the cell surface throughout the PSM (see B-B′′′′). |
|
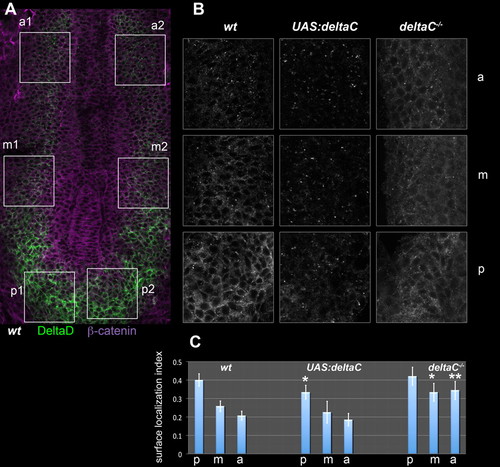
The subcellular localisation of DeltaD is controlled by DeltaC. (A) PSM of a flat-mounted wild-type (wt) zebrafish embryo immunostained for DeltaD (green) and β-catenin (magenta). Boxes show the left and right anterior (a), middle (m) and posterior (p) PSM regions that were sampled for quantitative analysis. (B) Enlargements of the corresponding boxed a, m and p regions from wild-type, DeltaC-overexpressing [heat-shocked Tg(UAS:dlc)cj2;Tg(hsp70l:Gal4vp16)vu22, ′UAS:deltaC′] and DeltaC-defective (dlctw212b/tw212b, ′deltaC-/-′) embryos; the DeltaD staining is shown in each case. (C) DeltaD surface localisation index computed for each region in each genotype. Values are means of n samples (left and right sides of n/2 embryos), where n=28 for wild type, n=10 for DeltaC overexpressing, and n=20 for DeltaC defective. Error bars show 95% confidence intervals. *, P<0.01; **, P<0.0001; versus the corresponding region of the wild type (one-tailed t-test). |