- Title
-
Zebrafish her8a is activated by Su(H)-dependent Notch signaling and is essential for the inhibition of neurogenesis
- Authors
- Chung, P.C., Lin, W.S., Scotting, P.J., Hsieh, F.Y., Wu, H.L., and Cheng, Y.C.
- Source
- Full text @ PLoS One
|
her8a expression in the developing zebrafish embryos. her8a expression is restricted in the developing nervous system during zebrafish embryogenesis analyzed by in situ hybridization. Stages of embryos shown in bottom left corner of each panel. Yolks were removed and embryos were flat-mounted, dorsal view except F and G lateral. her8a expression first appears in the developing brain at bud stage (A) and later becomes restricted to specific brain areas (B?E). The transcripts can be detected in the spinal cord from the 3-somite stage (3ss) (B) and retained until the latest stage analyzed (48 hpf) (C?G). Dashed circles in D mark the otic vesicles. Insert panel in D is an enlargement of the hindbrain, and in E is an enlargement of the eye. Arrowheads in D and E indicate her8a expressing cells at the midline. No signal was detected using sense riboprobe (C′). (H) her4 expression at 48 hpf . fmb, forebrain?midbrain boundary; hb, hindbrain; hp, hypothalamus; le, lens; mb, midbrain; r1-r7, rhombomere 1?7; rn, retinal neuroepithelial cells; sc, spinal cord; tel, telencephalon. EXPRESSION / LABELING:
|
|
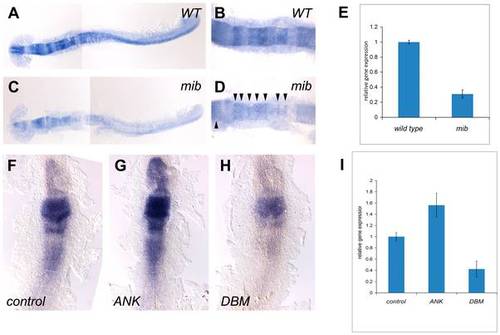
her8a is regulated by the Su(H)-dependent Notch signaling pathway. her8a is expressed at lower levels in the Notch activity-deficient mind bomb mutant embryos (C and D) in comparison to wild-type siblings (A and B). B and D are higher power views of hindbrain regions in A and C, respectively. Note the remnants of her8a transcripts in mind bomb mutants at the midbrain and hindbrain rhombomere boundaries (arrowheads in D). (E) Quantification of her8a expression analyzed by qPCR showing significantly reduced level of her8a in mind bomb mutants. (F?H) RNA encoding constitutively-active Su(H) (ANK) (G) or dominant-negative Su(H) (DBM) (H) was injected at the one or two-cell stage and analyzed at the 3-somite stage. (F) Control embryos injected with GFP mRNA; (G) her8a expression is upregulated by constitutive-active Su(H) injection; (H) her8a expression is downregulated in dominant-negative Su(H) injected embryos. (I) The levels of her8a expression in Su(H) variant injected embryos were further quantified by qPCR, showing significantly increased her8a expression in ANK injected embryos and decreased expression in DBM over-expressed embryos. ANK, constitutive-active Su(H); DBM, dominant-negative Su(H), mib: mind bomb mutants. |
|
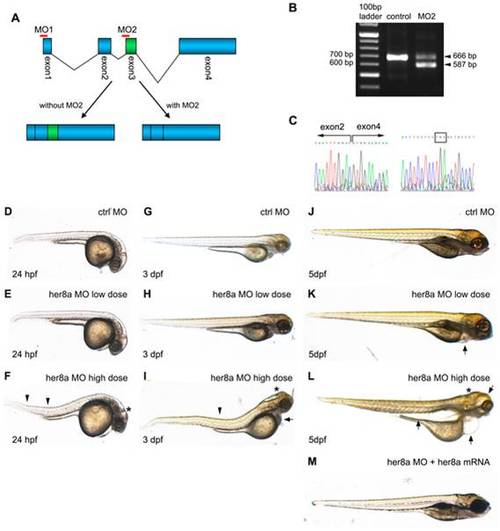
Knockdown of Her8a by specific morpholino antisense oligonucleotides causes developmental abnormalities. (A) Schematic representation shows the genomic organization of the her8a gene. Regions targeted by translational-blocking (MO1) and splice-blocking (MO2) morpholinos are shown. (B) The efficacy of MO2 was validated by RT-PCR using primers as indicated in A. Wild-type her8a mRNA produces a 666 bp PCR product while alternatively spliced transcripts from morphant embryos yield a 587 bp fragment. (C) The mis-spliced event resulted in loss of exon 3 confirmed by sequencing the 587 bp PCR product in B (left panel). The mis-splicing event resulted in a premature stop codon in exon 4 (boxed, right panel). (D?M) Representative images of morpholino injected embryos. (D, G and J) Control morpholino; (E, H and K) ?low dose? indicates 6 ng of MO1 or 4 ng of MO2 injection, resulting in indistinguishable phonotype; (F, I and L) ?high dose? represents 12 ng MO1 or 8 ng of MO2 injection, which resulted in identical morphological defects. Arrows denote bent body characteristics, and arrowheads indicate edemas in eyes, pericardial sac and abdominal cavity. Malformation of brains is marked by asterisks. (M) These morphological phenotypes can be rescued by concomitant injection of 2 ng her8a mRNA. |
|
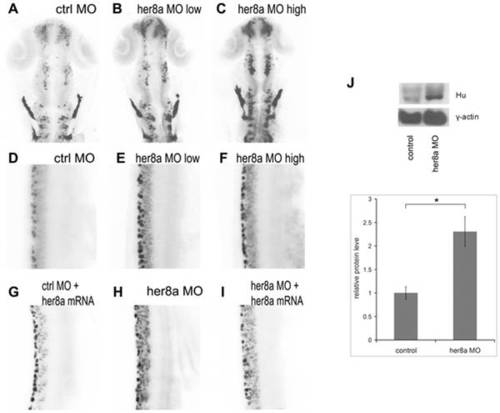
Her8a morphants exhibit upregulation of HuC/D expression. HuC/D expression is upregulated in Her8a morpholino injected embryos analyzed by immunohistochemistry with anti-Hu antibody at 24 hpf. The black-and-white fluorescent signals were inverted to negative film for a better presentation. (A and D) embryos injected with control morpholino. (B and E) 6 ng of MO1 or 4 ng of MO2 injection (low dose) resulted in indistinguishable phonotype and therefore only embryos injected with 4 ng of MO2 are shown (see Fig. S2 for MO1). (C and F) Injection of 12 ng MO1 or 8 ng MO2 (high dose) resulted in identical morphological defects and only MO2 injected embryos are shown, which shows more dramatic upregulation of HuC/D expression. (A?C) Brain regions; (D-I) 3-somite to 9-somite level of the spinal cord. (A?F) Hu-positve cells were significantly increased in the morpholino injected embryos. (G?I) The phenotype can be rescued by co-injection of morpholino with her8a mRNA. (J) Western blot analysis confirming the levels of HuC/D expression in Her8a morphants were up-regulated in comparison to the control. * p<0.05. EXPRESSION / LABELING:
|
|
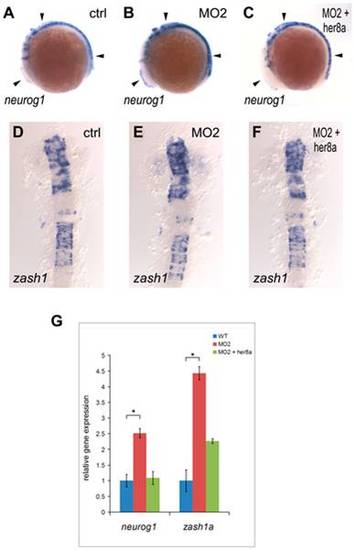
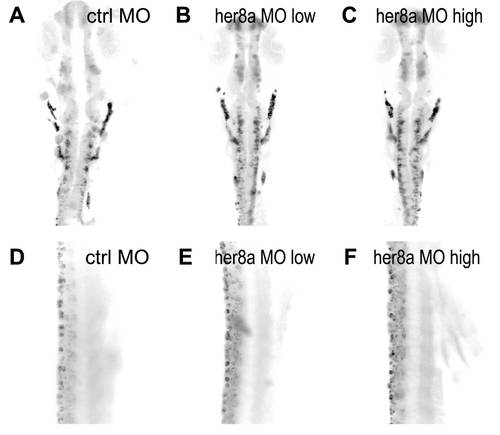
Increased neuronal precursors in Her8a knockdown embryos. Proneural genes were upregulated in the morpholino injected embryos and this effect was rescued by co-injection with her8a mRNA. Proneuronal markers neurogenin1 (A?C) and zash1 (D?F) were analyzed by in situ hybridization. Embryos were injected with control morpholino (A and D), 8 ng of MO2 (B and E), or co-injection of 8 ng of MO2 with 480 pg her8a mRNA (C and F). The most dramatic phenotypes are indicated by arrows. Stages of embryos are 6-somite stage (A-C) and 16-somite stage (D?F). (G) qPCR analysis showing the levels of neurogenin1 and zash1 are both significantly increased in embryos injected with her8a morpholino. * p<0.05. EXPRESSION / LABELING:
|
|
Glial precursors and mature glial cells were reduced in Her8a knockdown embryos. Her8a morpholino injection downregulates the expression of the glial precursor marker slc1a3a (A, B) and radial glial markers (gfap for radial glial cell body (D, E) and zrf-1 for glial fibers (G, H)), and this effect was rescued by co-injection with her8a mRNA (C, F, I). (A, D, G) Control morpholino, (B, E, H) 8 ng of MO2, (C, F, I) Co-injection of 8 ng of MO2 with 0.5 ng her8a mRNA. Embryos were harvested at the 18-somite stage (A?C) and 36 hpf (D?I). (A?F) in situ hybridization; (G?I) immunohistochemistry with zrf-1 antibody, fluorescent signals were inverted to negative film for a better presentation. (J) qPCR analysis showing the levels of slc1a3a and zash1 and zrf-1 are significantly decreased in embryos injected with her8a morpholino. * p<0.05. EXPRESSION / LABELING:
PHENOTYPE:
|
|
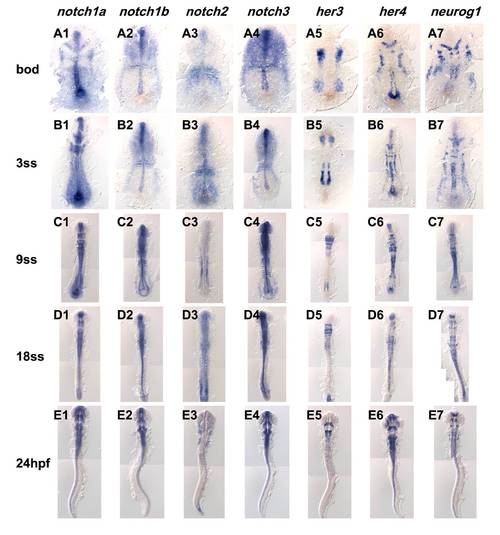
Expression comparison of notch homologues, her3, her4 and neurogenin1. Expression of notch homologues and known Notch target, her3 and her4, and neurogenenin1 analyzed by in situ hybridization. Name of the gene analyzed shown on the top row and stages of embryos shown on the left column. Embryos were flat-mounted, dorsal view. |
|
Embryos injected with MO1 or MO2 resulted in very similar morphological phenotypes. (A, B) Embryos injected with control morpholino analyzed at 5 days post fertilization. (C, D) Injection of 6 ng of MO1 or 4 ng of MO2 (lower dosage) caused very similar phenotype exhibiting pericardial edema. (E, F) Embryos injected with 12 ng MO1 or 8 ng of MO2 (higher dosage) show an identical phenotype including brain malformation and edemas in eyes, pericardial sac and the abdominal cavity. |
|
Embryos injected with MO1 exhibit upregulation of HuC/D expression. (A, B) Embryos injected with control morpholino. (C, D) Injection of 6 ng of MO1 (low dose) resulted in upregulation of HuC/D expression (E, F) 12 ng of MO1 injection (high dose) displayed more dramatic upregulation of HuC/D. |
|
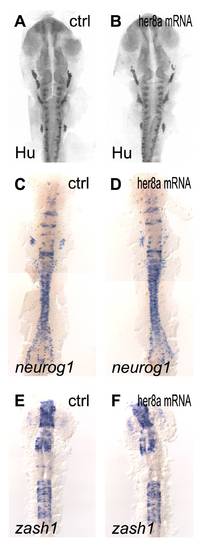
Over-expression of her8a mRNA did not alter the expression of proneural and pan-neuronal markers. Embryos were analyzed by immunohistochemistry with HuC/D antibody (A, B) and in situ hybridization with neurogenin1 (C, D) or zash1 (E, F). (A, C, E) Embryos were injected with GFP mRNA as control. (B, D, F) Injection of her8a mRNA revealed no significant deviation. |
|
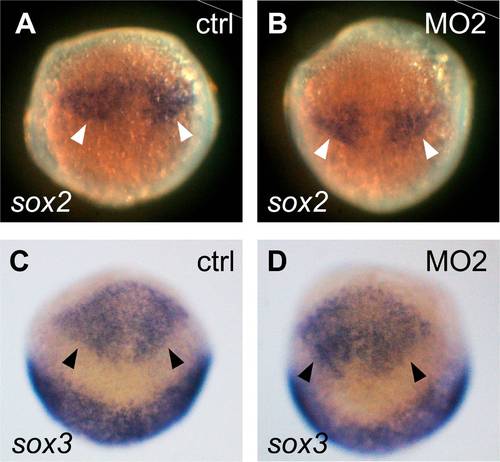
No significant alteration can be detected in sox2 or Sox3 expressing neural progenitors in Her8a morphants. Embryo injection with control morpholino (A and C) or 8 ng of MO2 (B and D) analyzed with sox2 (A and B) or sox3 (C and D) riboprobes. sox2 and sox3 were expressed in the neural precursors located within the neuroectodermal region (arrowheads). 75% epiboly; dorsal view, animal pole toward the top. |
|
The phenotypes in Her8a morphants were not caused by none specific p53 activation. Embryos co-injected her8a MOs with p53 MO (C, F, I, L, O, R) were compared to Her8a morphants (B, E, H, K, N, Q) and did not cause any detectable deviation analyzed with all markers tested. (A, D, G, J, M, P) Embryos injected with control morpholino. |