- Title
-
A beta-Catenin-Independent Dorsalization Pathway Activated by Axin/JNK Signaling and Antagonized by Aida
- Authors
- Rui, Y., Xu, Z., Xiong, B., Cao, Y., Lin, S., Zhang, M., Chan, S.C., Luo, W., Han, Y., Lu, Z., Ye, Z., Zhou, H.M., Han, J., Meng, A., and Lin, S.C.
- Source
- Full text @ Dev. Cell
|
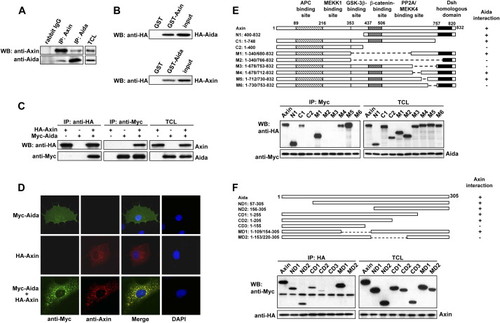
Identification of Aida as an Axin Interaction Partner (A) Endogenous Axin and Aida interact with each other. Axin and Aida in untransfected C2C12 cells were immunoprecipitated with rabbit anti-Axin and anti-Aida, respectively. Rabbit IgG was used as control. Detection of Axin and Aida in the immunoprecipitates and total cell lysates (TCLs) was carried out using anti-Axin and anti-Aida antibodies, respectively. (B) GST pull-down assay to evaluate Axin interaction with Aida in vitro. The ability of GST-Axin to retain Aida present in the cell lysate from HEK293T cells transfected with pCMV-HA-Aida was analyzed by western blotting with anti-HA (upper panel). GST-Aida specifically pulled down HA-Axin in the cell lysate (lower panel). In either case, GST alone did not interact with Aida or Axin. Input represented one-sixth of lysate used for GST pull-down. (C) Axin and Aida interact with each other when overexpressed in HEK293T cells. HA-Axin and Myc-Aida were expressed in HEK293T cells singly or in combination. Reciprocal coimmunoprecipitation was performed by using anti-HA or anti-myc antibody followed by immunoblotting using anti-Myc or anti-HA antibody as indicated. (D) Colocalization of Axin and Aida in mammalian cells. COS-7 cells were transiently transfected with myc-Aida and Axin and stained for Axin (red) and Aida (green) using rhodamine conjugated mouse antibody and FITC conjugated rabbit antibody, respectively. (E) Aida interacts with a specific C-terminal domain of Axin. Schematic diagrams depict different Axin deletion mutants used in the domain-mapping experiments. Different HA-tagged Axin deletion constructs were transiently transfected into HEK293T cells together with Myc-tagged Aida. Cell lysates were immunoprecipitated with anti-Myc antibody, followed by immunoblotting using anti-HA for Axin proteins and anti-Myc for Aida. (F) Determination of Axin binding sites in Aida. Shown on the top are schematic diagrams of different Aida deletion mutants used in the domain-mapping experiments. Same experiments were performed as described in (E). |
|
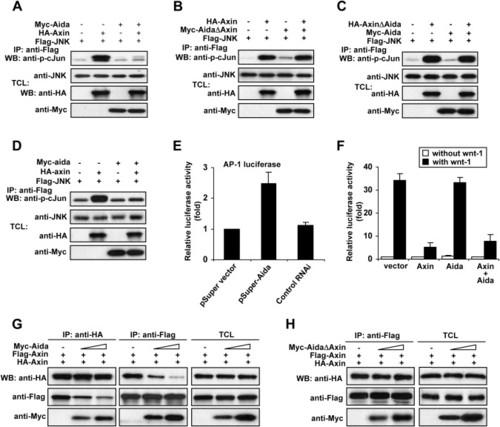
Aida Inhibits Axin-Meditated JNK Activation by Disrupting Homodimerization of Axin (A) Aida inhibits Axin-mediated JNK activation. HEK293T cells were transfected with FLAG-tagged JNK together with other plasmids as indicated. Following immunoprecipitation of FLAG-JNK, JNK kinase activities were assayed using GST-c-Jun as substrate. The amount of the kinase and the kinase activity in each immunoprecipitate were quantified by immunoblotting using anti-JNK antibody and anti-phospho-c-Jun antibody, respectively. (B) AidaΔAxin that cannot bind to Axin fails to attenuate Axin-mediated JNK activation. (C) AxinΔAida-mediated JNK activation could not be attenuated by Aida. (D) Zebrafish Aida inhibits Axin-induced JNK activation. An experiment similar to (A) was performed using zebrafish axin and aida instead of their mouse counterparts. The immunokinase assay was performed as described above. (E) The siRNA against Aida enhances AP-1 activity. HEK293T cells were transfected with AP-1 luciferase reporter together with empty pSUPER vector, pSUPER-Aida, or control RNAi. At 32 hr posttransfection, relative luciferase activity was measured. (F) Aida has no effect on Wnt signaling as assessed with LEF-1 luciferase reporter. HEK293T cells were transfected with 0.5 μg of LEF-1 luciferase reporter plasmid together with 1.5 μg of Axin, Aida alone, or both. Aida did not affect basal, wnt-1-induced, or Axin-downregulated reporter expression. (G) Aida disrupts Axin homodimerization as determined by using two differently tagged Axins, HA-Axin and FLAG-Axin. HEK293T cells were transfected with HA-Axin and FLAG-Axin together with increasing amounts of Aida (0 μg, 1 μg, and 3 μg). When HA-tagged Axin was immunoprecipitated by anti-HA, the amount of coprecipitated FLAG-tagged Axin was negatively correlated with the increasing amounts of cotransfected Aida (left panel); similarly, decreasing amounts of HA-tagged Axin were coprecipitated with FLAG-Axin by the anti-FLAG antibody in the presence of increasing amounts of Aida (middle panel). The right panel shows similar expression levels of HA- and FLAG-tagged Axin among the three samples, and it shows that Aida levels were proportionally increased when increasing amounts of DNA were transfected. (H) AidaΔAxin has no effect on Axin homodimerization. Coimmunoprecipitated HA-Axin levels in anti-FLAG immunoprecipitates were not changed in the presence of increasing amounts of AidaΔAxin. |
|
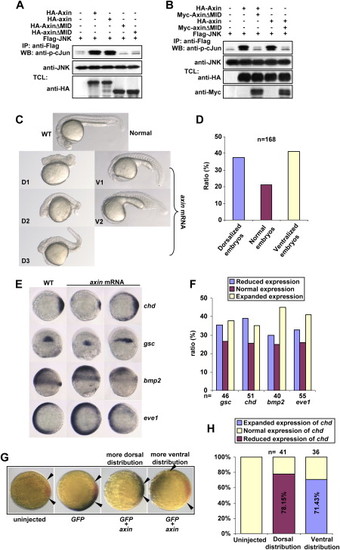
Axin Contains Intrinsic Dorsalizing Activity (A) Zebrafish axin and axinΔMID behave similarly to their mouse counterparts in JNK activation. HEK293T cells were transfected with FLAG-JNK together with other plasmids as indicated. Immunokinase assays were performed as described above. (B) Zebrafish axinΔMID is a dominant-negative form for axin-mediated JNK activation. HEK293T cells were transfected and followed by immunokinase assay at 36 hr posttransfection. (C) Injection of zebrafish axin mRNA induces opposing phenotypic changes in embryos at 24 hpf. axin mRNA was injected into one-cell embryos at a dose of 300 pg. Shown are lateral views of live embryos at 24 hpf. D1, D2, and D3 display characteristic dorsalized phenotypes at 24 hpf. D2 and D3 phenotypes of injected embryos showed shortened tail with the ventral tail fin missing. D1 phenotype was the most severe one. V1 and V2 phenotypes displayed loss of the head and notochord together with enlargement of the blood island. (D) Statistical data for (C). (E) Expression of marker genes in axin-injected embryos. Full-length axin mRNA (300 pg) was injected into one-cell embryos and followed by in situ hybridization at the shield stage. Embryos for chd and eve1 were animal pole views with dorsal to the right; embryos for gsc were dorsal views with animal pole to the top; and embryos for bmp2 were lateral views with dorsal to the right. (F) Statistical data for (E). (G) chd expression changes in an injection-position-dependent manner. Wild-type axin mRNA (250 pg) was coinjected with GFP mRNA (50 pg), and double in situ hybridization was performed at the shield stage for GFP(blue) and chd (red). The blue areas expressing GFP indicated the injection position. Note that when injected mRNAs were distributed into areas proximal to the dorsal side as judged at the shield stage, chd expression was weaker (shorter distance between arrows), whereas ventral distribution gave rise to enhanced expression of chd. (H) The percentage of embryos with reduced chd expression and dorsal distribution of injected GFP mRNA, and the percentage of embryos with enhanced chd expression and ventral distribution of GFP mRNA, were diagramed. |
|
Components of JNK Signaling Are Required for Axin-Induced Dorsalization (A) Injection with 50 pg of axinΔMID mRNA caused embryonic ventralization only. The top panel on the left shows an illustration of axinΔMID structure. Live ventralized embryos at 24 hpf lack the head and the notochord, but possess an enlarged blood island (indicated by an arrow). The right panel shows that axinΔMID overexpression inhibited gsc and chd expression (dorsal views) but expanded bmp2 and drl expression (animal pole views with dorsal to the right) at the shield stage. (B) Statistical data for axinΔMID overexpression. Upward and downward arrows indicated increased and decreased expression, respectively. (C and D) MKK4 siRNA, MKK4-KM (C), and JNK-APF (D) abolish axin-mediated JNK activation. FLAG-tagged JNK was cotransfected with different constructs as indicated on the top. JNK activities from different transfections were determined as described in Figure 2. (E?H) Percentages of dorsalized and ventralized embryos, judged by morphological changes at 24 hpf (E and G) or by marker changes at the shield stage (F and H). Injection dosages: axin mRNA, 200 pg; MKK4-KM mRNA, 300 pg; JNK-APF mRNA, 300 pg. |
|
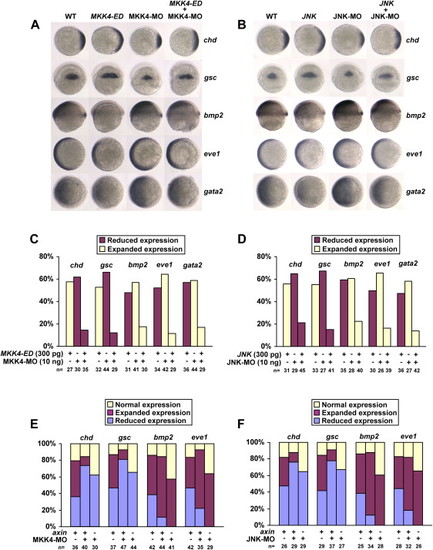
JNK Is Involved in Dorsoventral Patterning (A) Expression patterns of marker genes in wild-type embryos or embryos injected with 300 pg of MKK4-ED mRNA, 10 ng of MKK4-MO, or both. Embryos for chd and eve1 are animal pole views with dorsal oriented toward the right; embryos for gsc are in dorsal views with animal pole to the top; and embryos for bmp2 and gata2 are in lateral views with dorsal to the right. (B) Expression patterns of marker genes in wild-type embryos or embryos injected with 300 pg of JNK mRNA, 10 ng of JNK-MO, or both. Experiments are performed as described in (A). (C and D) Statistical data for (A) and (B), respectively. (E) Percentages of embryos with expression changes of marker genes in shield stage caused by axin mRNA injection or coinjection of axin and MKK4-MO. Approximately 200 pg of axin mRNA was injected into one-cell embryos alone or together with 10 ng of MKK4-MO. In situ hybridization analysis of different marker genes in injected embryos was performed. Percentages of embryos with elevated, normal, or reduced expression levels of the marker genes were calculated and diagramed. (F) Ratios of dorsalized and ventralized embryos derived from the same experiments as those detailed for (E), except that JNK-MO was used instead of MKK4-MO. |
|
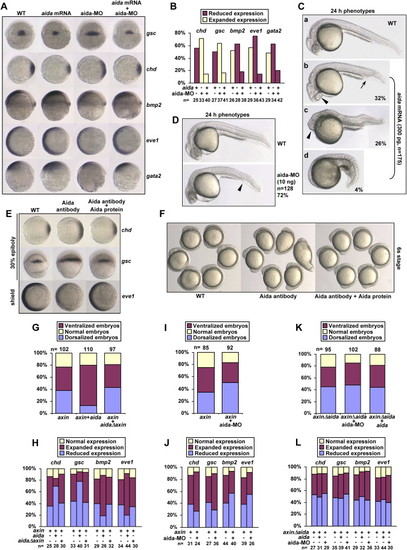
aida Induces Ventralization and Inhibits axin-Induced Dorsalization in Zebrafish Embryos (A) Effect of aida overexpression or knockdown on expression of the dorsal and ventral markers. Embryos were injected with 300 pg of aida mRNA or 10 ng of aida-MO and examined for marker expression at the shield stage by in situ hybridization. Ectopic expression of aida repressed expression of gsc and chd, but expanded the expression of bmp2, eve1, and gata2. Knockdown of aida by MO injection caused reversed effects, which could be attenuated by coinjection with 100 pg of aida mRNA. (B) Statistical data for (A). (C) aida-induced ventralization of various degrees at 24 hpf. (Ca) Uninjected embryos. (Cb?Cd) Embryos were injected with 300 pg of aida mRNA. Of the injected embryos, 32% showed an increased blood island (Cb, indicated by an arrow), short tail, and closer eyes (Cb, indicated by arrowhead); 26% showed an increased blood island (Cc, indicated by arrowhead), reduced head, and eye fusion; and 4% showed complete loss of head and notochord (Cd). (D) aida-MO causes dorsalization in embryos at 24 hpf. Embryos were injected with 10 ng of aida-MO. Arrowhead marks a reduced blood island. (E) Depletion of aida protein by antibody injection leads to formation of dorsalized embryos. One-cell embryos were similarly injected with affinity-purified anti-Aida polyclonal antibody or with the antibody preincubated with recombinant aida protein, and in situ analysis of the expression of chd and gsc in 30% epiboly embryos, and eve1 in shield-stage embryos, was performed. (F) One-cell embryos were injected as in (D). Photos were taken at the six-somite stage. (G) Statistical data for aida inhibition of axin-induced dorsalization. Injection with 300 pg of aida mRNA, but not with 300 pg of aidaΔAxin mRNA, decreased the percentage of 24 hpf embryos that were dorsalized by injection of wild-type axin. (H) Ratios of embryos with normal, expanded, or reduced expression of various markers indicated, after injection of axin mRNA, coinjection of axin with aida, or aidaΔaxin mRNA. (I) aida-MO increases the ratio of dorsalized embryos induced by axin. axin mRNA (300 pg) and 10 ng of aida-MO were injected either alone or together. The ratios of different embryos were calculated based on morphological observations of embryos at 24 hpf. (J) Ratios of different embryos determined by in situ analysis of marker genes at the shield stage after injection of axin mRNA (300 pg) or coinjection of axin with aida-MO (10 ng). (K) Statistical data for the effect of aida mRNA or aida-MO on axinΔaida-induced dorsalization. Injection with 300 pg of aida mRNA or 10 ng of aida-MO had no significant effect on the percentage of 24 hpf embryos that were dorsalized by injection of axinΔaida. (L) Ratios of embryos with normal, expanded, or reduced expression of various markers indicated, after injection of axinΔaida mRNA, coinjection of axinΔaida with aida mRNA (300 pg), or coinjection of axinΔaida with aida-MO (10 ng). |
|
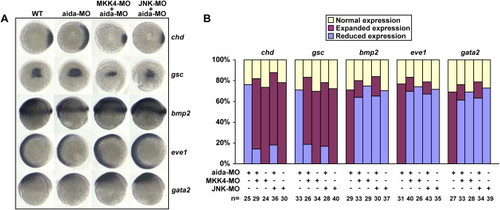
Coinjection of MKK4-MO or JNK-MO Could Cancel the Dorsalizing Effect of aida-MO (A) Expression patterns of marker genes in wild-type embryos or embryos injected with 10 ng of aida-MO, 10 ng of MKK4-MO, 10 ng JNK-MO, or the aforementioned in combination. (B) Statistical data for (A). Injection of 300 pg of aida mRNA induced ventralization, while injection of 10 ng of aida-MO had the opposite effect. The dorsalized phenotype could be rescued by coinjection of aida mRNA. EXPRESSION / LABELING:
|
|
Wnt Signaling-Independent Regulation of Dorsoventral Patterning by the Axin/JNK Signaling Cascade (A) Genetic interaction between μ-catenin and axin. Ten nanograms of μ-cat2-MO was injected into one-cell embryos alone or together with 300 pg axin mRNA or 150 pg axinΔMID mRNA. In situ hybridization of marker genes was performed at the shield stage. (B) JNK mRNA, JNK-MO, aida mRNA, aida-MO, or aida antibody was injected into one-cell embryos, and expression of bozozok was analyzed in sphere-stage embryos. (C) JNK mRNA, JNK-MO, and wnt8-MO were injected singly or in combination as indicated. In situ analysis of tbx6 expression was performed at the shield stage. (D) JNK signaling has no effect on Wnt signaling as assessed with LEF-1 luciferase reporter and β-catenin stability assay. HEK293T cells were transfected with 0.5 μg of LEF-1 luciferase reporter plasmid together with 1.5 μg of MKK4, JNK, MKK4-KM, JNK-APF, or MKK4-RNAi alone or in combination for the LEF-1 luciferase activity. None of the plasmids affected basal or wnt-1-induced reporter expression. For the β-catenin stability assay, HEK293T cells were transfected with 4 μg of MKK4, JNK, MKK4-KM, JNK-APF, or MKK4-RNAi alone or in combination together with 0.5 μg of GFP, 1 μg of Myc-β-catenin, and 1.5 μg of wnt-1. Results showed that JNK signaling did not affect the β-catenin protein level. (E) A model for dual activities of Axin in dorsal development of vertebrate embryos. In the canonical Wnt pathway, μ-catenin promotes dorsalization. Axin reduces μ-catenin levels by serving as a scaffold for the assembly of μ-catenin degradation complex, and hence attenuates dorsalization. Axin also exerts an opposing role, i.e., promotion of dorsalization, by means of activation of JNK. Aida, by virtue of repressing Axin-induced JNK activation through disrupting Axin homodimerization, attenuates Axin-enhanced dorsalization. |
|
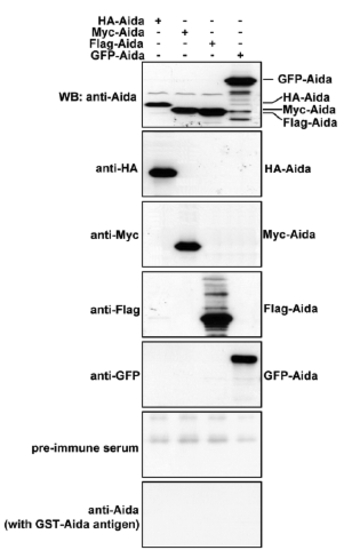
Characterization of Aida polyclonal antibody. Differently tagged Aida plasmids were separately transfected into 293T cells. Protein samples from the differently transfected cells were subjected to immunoblotting with anti-HA, anti-Myc, anti-Flag, anti-GFP or anti-Aida antibodies to detect Aida expression. Anti-Aida recognized the same protein band as those detected by the respective tag antibodies. No specific band was detected with pre-immune serum or after blocking with Aida protein. |
|
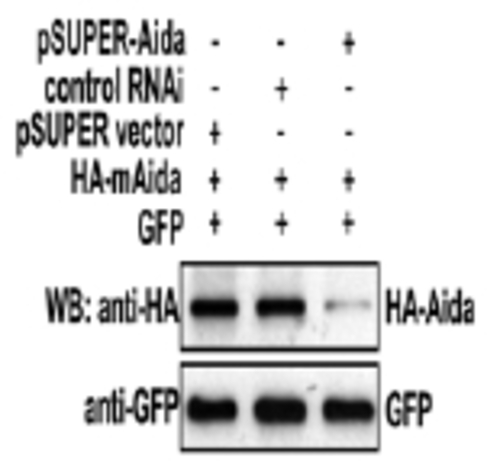
Characterization of pSUPER-Aida. About 0.3 μg of HA-Aida expression plasmid was co-transfected with 8 μg of pSUPER vector, control RNAi or pSUPER-Aida into HEK 293T cells; pEGFP was co-transfected as internal transfection control. At 30 h posttransfection, cells were lysed and immunoblotting was carried out with anti-HA antibody to compare the Aida protein levels int eh presence or absence of pSUPER-Aida |
|
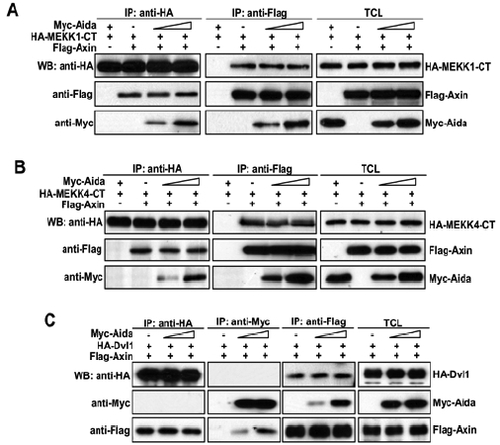
Aida does not affect binding affinities between Axin and MEKK1-CT, MEKK4-CT or disheveled. (A) Cells were transfected with HA-MEKK1-CT and FLAG-Axin together with increasing amount of Myc-Aida (0, 1, 3 μg). Immunoprecipitation and western blotting using different antibodies were carried out as above described. (B and C) Similar experiments to that in A were performed except for the use of HA-MEKK4-CT (B) and HA-Dvl (C). |
|
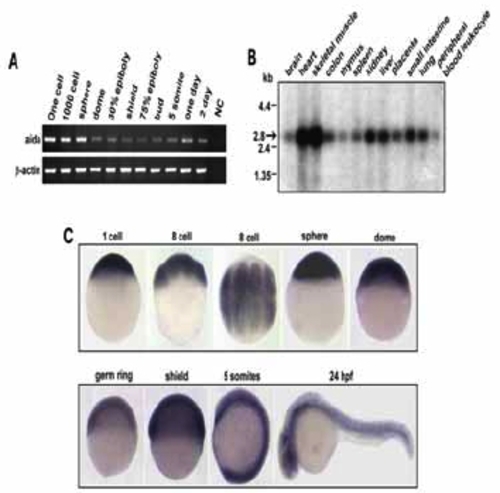
Temporal and spatial expression of aida. (A) Developmental expression pattern of aida in zebrafish was determined by reverese transcription polymerase chain reaction (RT-PCR). RNA sample from one-cell embryos with no addition of reverse transcriptase was used as negative control Zebrafish β-actin was the loading control. (B) Northern blot with poly(A)+ RNA isolated from various tissues of human, indicated on the top, was hybridized with 32P-labelled human AIDA DNA probe. the band representing Aida was indicated by an arrow. (C) Spatiotemporal expression of zebrafish aida was detected by whole-mount in situ hybridization at different stages at indicated stages. Note that aida is ubiquitously expressed throughout these stages. |
|
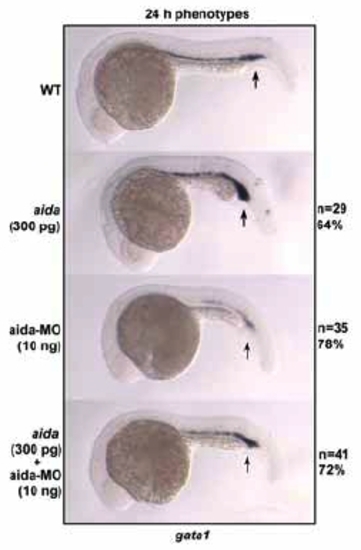
Aida is required for expression of the ventral hematopoietic marker gata1. Embryos were injected with 300 pg of aida mRNA or 10ng of aida-MO. Ectopic expression of aida repressed gata1 expression in the blood island (indicated by arrows) at 24 hpf, while aida-MO caused a reversed effect. EXPRESSION / LABELING:
|
Reprinted from Developmental Cell, 13(2), Rui, Y., Xu, Z., Xiong, B., Cao, Y., Lin, S., Zhang, M., Chan, S.C., Luo, W., Han, Y., Lu, Z., Ye, Z., Zhou, H.M., Han, J., Meng, A., and Lin, S.C., A beta-Catenin-Independent Dorsalization Pathway Activated by Axin/JNK Signaling and Antagonized by Aida, 268-282, Copyright (2007) with permission from Elsevier. Full text @ Dev. Cell