- Title
-
New functions for a vertebrate Rho guanine nucleotide exchange factor in ciliated epithelia
- Authors
- Panizzi, J.R., Jessen, J.R., Drummond, I.A., and Solnica-Krezel, L.
- Source
- Full text @ Development
|
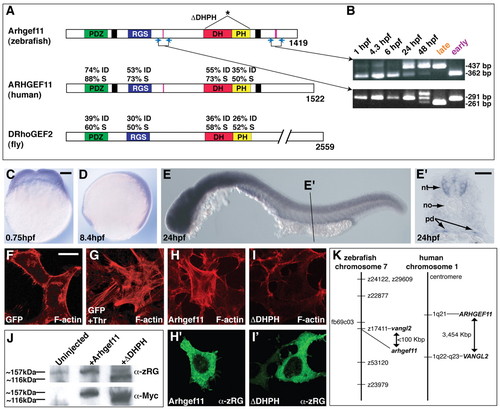
Zebrafish Arhgef11 is a homolog of human ARHGEF11 and is expressed during early embryogenesis. (A) Schematic depicting the percentage of identical (I) and similar (S) amino acids between known functional domains of zebrafish Arhgef11 and its human and fly homologs. An asterisk marks the domains omitted in the dominant-negative construct (ΔDHPH). (B) Ethidium bromide stained agarose gel showing RT-PCR products after amplification of the indicated fragments (blue arrows in A) at the listed developmental times, along with fragments amplified from `early' and `late' cloned constructs. Alternatively-spliced exons are indicated by pink bars in A. (C-E) WT embryos after whole-mount in situ hybridization using antisense probe for arhgef11 at the indicated developmental stages. Scale bar, 100 μm. (E') Cryosection through the indicated region in E. Abbreviations: nt, neural tube, no, notochord, pd, pronephric ducts. Scale bar, 50 μm. (F-I') Serum-starved cultured HEK293 cells expressing GFP, myc-Arhgef11, or myc-ΔDHPH. Cells in G were incubated with thrombin. F-actin is visualized by phalloidin in red (F-I), and Arhgef11 or ΔDHPH constructs are in green (H' and I', respectively) as detected by α-zRG. Scale bar, 20 μm. (J) Western blots using protein extracts from 6 hpf WT embryos injected as indicated. α-zRG detects both endogenous and overexpressed forms of full-length Arhgef11 (∼157 kDa) and overexpressed ΔDHPH (∼116 kDa), whereas &alpha-Myc only detects the overexpressed forms of each. (K) Schematics of chromosomal locations of zebrafish and human arhgef11 genes, showing synteny between the two chromosomes in the region encompassing arhgef11. |
|
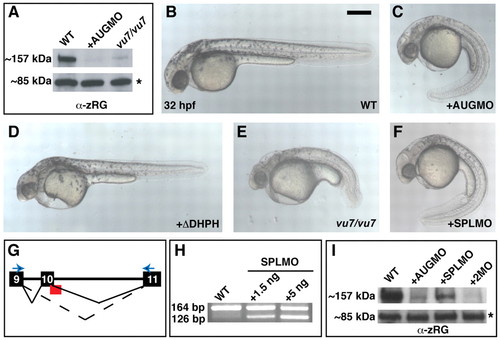
Interference with Arhgef11 function yields morphological defects in zebrafish embryos. (A) Western blot using α-zRG on extracts prepared at 13 hpf from the indicated embryos. The asterisk marks expression of an unidentified 85 kDa protein also detected by α-zRG. (B-F) Live embryos at 32 hpf: uninjected WT (B), WT injected with 4 ng MOAUG (AUGMO; C), WT injected with 350 pg of RNA encoding ΔDHPH construct (D), vu7/vu7 mutants (E) and WT injected with 5 ng MOSPL (SPLMO) (F). Scale bar, 300 μm. (G) Schematic depicting the MOSPL-binding site (red) at the exon 10-intron 10 boundary, with normal (solid line) and disrupted (dotted line) splicing shown. (H) Ethidium bromide stained agarose gel of RT-PCR products amplified from embryos after the indicated treatment using the primers represented by the blue arrows in G. (I) Western blot using α-zRG on extracts prepared at 13 hpf from uninjected WT, or WT after injection with 4 ng MOAUG, 5 ng MOSPL, or co-injected with both. EXPRESSION / LABELING:
|
|
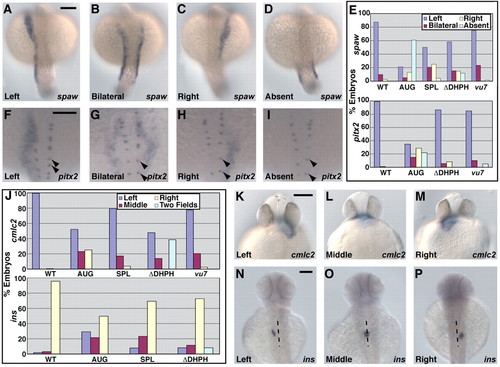
Loss of Arhgef11 function leads to abnormal expression of laterality markers. (A-D) Expression of spaw in 19- to 22-somite stage (∼19 hpf) embryos detected by in situ hybridization with antisense RNA probe. Scale bar, 150 μm. (E) Bar graph of the percentage of WT and treated embryos exhibiting the expression patterns for spaw shown in A-D, or pitx2 shown in F-I. (F-I) Expression of pitx2 in 22- to 25-somite stage (∼21 hpf) embryos detected by in situ hybridization with antisense RNA probe. Expression of pitx2 in Rohon Beard cells (marked by arrowheads) is not disrupted. Scale bar, 150 μm. (J) Graphical representation of the percentage of embryos exhibiting the expression patterns for cmlc2 shown in K-M or for ins shown in N-P. (K-M) Expression of cmlc2 in ∼33 hpf embryos detected by in situ hybridization with antisense RNA probe. Scale bar, 150 μm. (N-P) Expression of ins in ∼53 hpf embryos detected by in situ hybridization with antisense RNA probe. A dotted line marks the approximate midline. Scale bar: 150 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
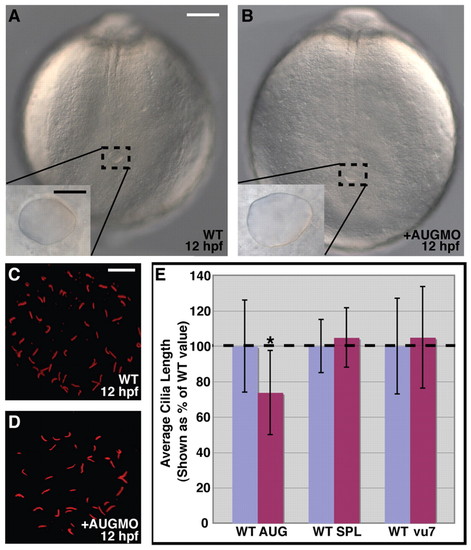
Arhgef11 is not required for formation of Kupffer′s vesicle and cilia. (A,B) Posterior region of live, uninjected WT and MO-injected embryos. The inset is a magnified view of Kupffer′s vesicle. White scale bar, 100 μm; black scale bar, 25 μm. (C,D) Acetylated tubulin immunostaining of cilia within Kupffer′s vesicle. Scale bar, 10 μm. (E) Comparison of Kupffer′s vesicle cilia length for WT control, MO-injected (AUG, SPL) and vu7/vu7 embryos. The asterisk marks significantly different lengths (P≤0.001). PHENOTYPE:
|
|
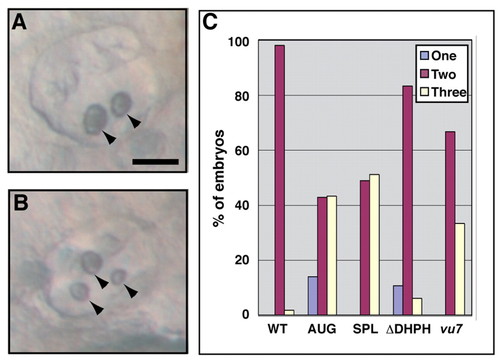
Arhgef11 function is important for proper otolith development. (A,B) Otic vesicles of example embryos at ∼48 hpf showing two or three otoliths (arrowheads). Scale bar, 50 μm. (C) Bar graph of the percentage of WT control, MO-injected, ΔDHPH-overexpressing, and mutant embryos with two otoliths in both otic vesicles, or with an abnormal number (one or three) in at least one otic vesicle. PHENOTYPE:
|
|
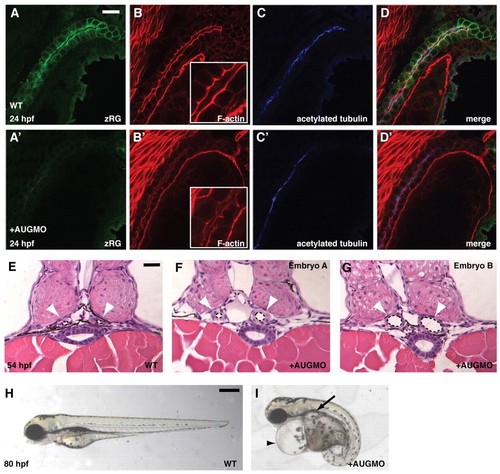
Loss of Arhgef11 function leads to formation of pronephric cysts. (A-D′) Fluorescent immunostaining of 24 hpf WT control and MOAUG-injected embryos to visualize zRG (green), F-actin (red) with magnified view inset, and acetylated tubulin (blue). Scale bar, 20 μm. (E-G) JB-4 sections of 54 hpf WT control embryos (E) compared with sections from two embryos injected with 4 ng MOAUG (F,G) at the same stage. Pronephric ducts are indicated by white arrowheads, and their lumens are outlined by dotted lines. Scale bar, 25 μm. (H,I) Live embryos at 80 hpf showing an uninjected WT embryo (H) compared with WT injected with 4 ng MOAUG (I), which exhibits cardiac edema (arrowhead) and pronephric cysts (arrow). Scale bar: 300 μm. PHENOTYPE:
|
|
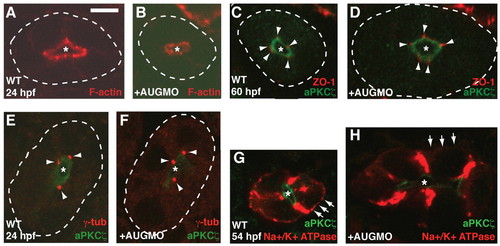
Apical-basal distribution of Na+/K+-ATPase is disrupted in morphants. Cryosections through pronephric ducts of uninjected WT (A,C,E,G) and MOAUG-injected (B,D,F,H) embryos at the indicated developmental times. The lumen (apical) is indicated by an asterisk, and the basal sides of ducts are outlined by dotted lines in A-F. (A,B) Filamentous actin in the pronephric ducts (red). (C,D) Cell junctions are stained with ZO1 antibody (red, arrowheads), and the apical region of the cells is marked by aPKCζ (green). (E,F) Microtubule-organizing centers are stained with anti-γ-tubulin (red, arrowheads), and the apical region of the cells is marked by aPKCζ (green). (G,H) Localization of Na+/K+-ATPase (red); the apical region of the cells is marked by PKCζ (green). Arrows highlight the presence of staining in basal regions of WT control cells and its absence in morphants. Scale bar: 5 μm. |
|
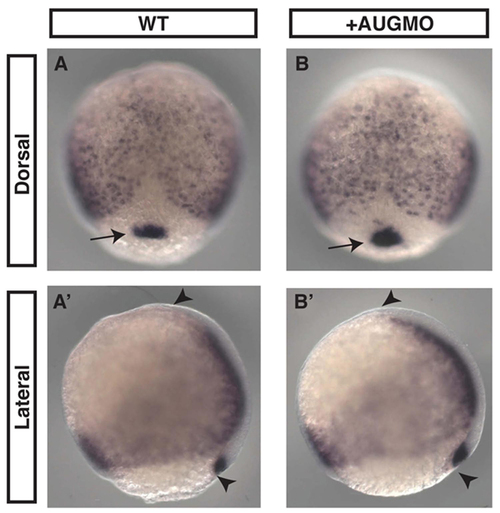
Endoderm and dorsal forerunners cells, which ultimately form Kupffer's vesicle, appear normal in morphants. In situ hybridization using sox17 probe on 8.5 hpf uninjected WT (A,A′) and MOAUG-injected (B,B′) embryos demonstrates formation of endoderm and dorsal forerunner cells (arrows) is not disrupted in morphants. Embryo length (marked by arrowheads) is also unaffected. Probe used was as described in Alexander and Stainier (Alexander and Stainier, 1999). EXPRESSION / LABELING:
|
|
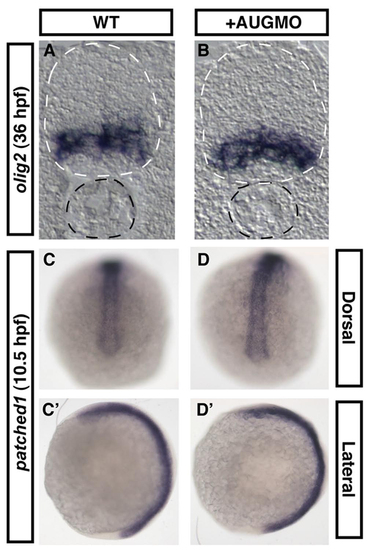
Midline structures and patterning are not impaired by Arhgef11 loss-of-function. In situ hybridization using olig2 probe on cryosections of WT control (A) and MOAUG-injected (B) embryos indicates normal patterning of the neural tube (outlined by white dotted lines). Notochord also appears normal (outlined by black dotted lines). In situ hybridization using probe for the Hedgehog target gene, patched1, on WT (C,C2) and morphant embryos (D,D2) also reveals no abnormalities. Probes for olig2 and patched1 were as described by Park et al. (Park et al., 2002) and Concordet et al. (Concordet et al., 1996), respectively. EXPRESSION / LABELING:
|

Unillustrated author statements EXPRESSION / LABELING:
|