- Title
-
Medial floor plate formation in zebrafish consists of two phases and requires trunk-derived Midkine-a
- Authors
- Schäfer, M., Rembold, M., Wittbrodt, J., Schartl, M., and Winkler, C.
- Source
- Full text @ Genes & Dev.
|
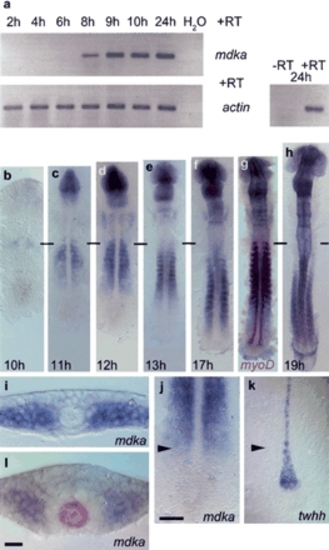
Mdka expression during zebrafish embryogenesis. (a) RT-PCR analysis: mdka transcription is first detected at 8 hpf (h); actin expression was used for calibration. -RT control using actin primers without addition of reverse transcriptase. (b-h) RNA in situ hybridization on 10-19-h embryos showing advancing mdka transcription in the paraxial mesoderm; horizontal bars mark the position of the first somite. (g) Double staining with myoD at 17 h shows progressive loss of mdka transcription in anterior somites. (i) mdka is expressed at the six-somite (6s; 12 hpf) stage in cells of the unsegmented paraxial mesoderm when convergence of the neural plate has just started. (j,k) The front of advancing mdka expression (j) demarcates the position of twhh restriction in MFP precursors (k) as indicated by arrowheads that also show level of transverse section in i. (l) At later stages, mdka is expressed in paraxial mesoderm and also the anterior neural keel, but excluded from a ring of cells surrounding the shh-positive notochord and MFP (8s stage, shh labeled in red). In dorsal views, anterior is to the top in b-h,j,k. Bars: j, 10 µm; l, 50 µm. EXPRESSION / LABELING:
|
|
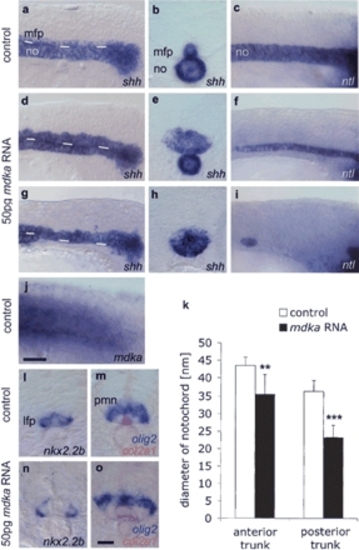
Mdka overexpression enlarges the MFP at the expense of the notochord. Lateral views (a,c) and transverse sections (b, posterior trunk) of noninjected 14s control embryos. (d-f) In embryos injected with 50 pg mdka RNA, the MFP is enlarged (expanded shh in MFP, n = 165/191, 86%; f-spondin, in n = 36/46, 76%; col2a1, n = 134/145, 92% [data not shown]) and the notochord is reduced (reduced shh in the notochord in n = 20/38, 53%; col2a1 in n = 27/88, 31%; and ntl in n = 11/45, 24%). (g-i) Severely affected embryo with complete block of notochord formation in the posterior trunk (lacking shh expression in n = 16/38, 42%; col2a1 in n = 41/88, 46%, and ntl in n = 30/45, 67%). White lines in a, d, and g indicate border between the notochord and MFP. Sections in b, e, and h are at similar positions in the posterior trunk. (j) Endogenous mdka transcription in the paraxial mesoderm extends up to the tailbud. (k) mdka overexpression resulted in a significant decrease of notochord width: n = 10; (**) p < 0.01 versus control for anterior trunk; (***) p < 0.001 versus control for posterior trunk; Mann-Whitney U-test (see Supplementary Fig. S3). (l-o) Transverse sections of 18s embryos showing enlarged MFP (expanded col2a1 in n = 37/44, 84% [o]), but normal expression of nkx2.2b in the lateral floor plate (n = 27/29, 93% [n]) and olig2 in motoneuron domain (n = 43/44, 98% [o]; n = 10, p = 0.796; see Supplementary Fig. S1) in mdka-injected embryos. Anterior is left in lateral views. Bars: j, 20 μm; o, 10 μm. (lfp) Lateral floor plate, (mfp) medial floor plate, (no) notochord, (pmn) motoneuron progenitor domain. EXPRESSION / LABELING:
|
|
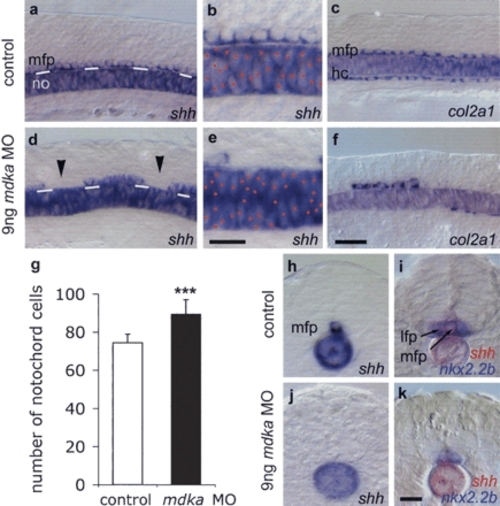
MFP formation requires Mdka protein. Lateral views of noninjected control (a-c) and 9 ng mdka MO morpholino-injected 12s embryos (d-f). mdka MO-injected embryos show gaps in the MFP (arrowheads; reduced MFP expression of shh in n = 103/136, 76% of injected embryos [d,e]; col2a1 in n = 114/188, 61% [f]; and f-spondin in n = 19/30, 63% [data not shown]) and increased cell density in the notochord (expressing shh [d,e] and ntl, n = 17/27, 63% [data not shown]). White lines in a and d indicate the border between the notochord and MFP. Red dots mark notochord cells in b and e. Large gaps were also observed in the hypochord (col2a1 reduced in n = 146/188, 78% of embryos [f]). (g) Mdka MO significantly increased the number of notochords cells: n = 10; (***) p < 0.001 versus control; Mann-Whitney U-test (see Supplementary Fig. S5). (h-k) Cross-sections of control (h,i) and mdka MO-injected (j,k) embryos at the trunk level showing loss of shh-positive MFP cells and fusion of nkx2.2b-expressing lateral floor plate cells (in n = 13/22, 59% of embryos). In several cases (n = 9/22, 41%) also lateral floor plate cells were missing, possibly due to insufficient levels of Shh. Bar: f, 20 μm; b,k, 10 μm. (hc) hypochord. EXPRESSION / LABELING:
|
|
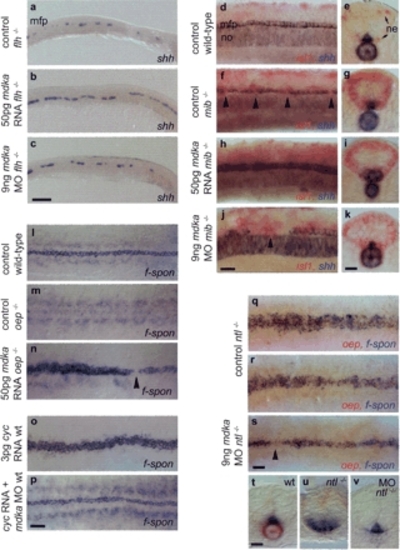
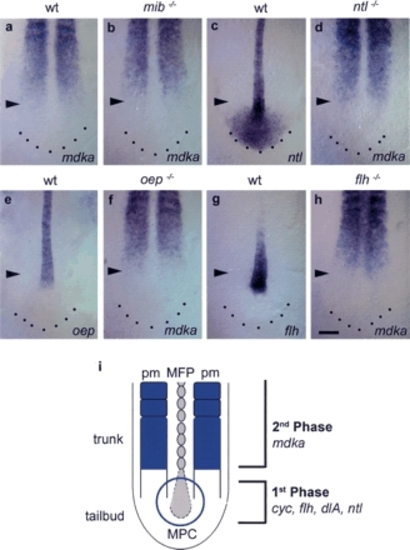
Epistatic interactions of mdka. (a,c) In floating head mutants (flh-/-) that lack a notochord and only retain islands of MFP cells (a), mdka overepression partially rescues the MFP defect. (a,b) Cell number is increased, resulting in an almost continuous line one cell in diameter (n = 21/27, 78%). Knockdown has no effect on MFP cell number and does not rescue the notochord (n = 42, shh [c]; n = 18, ntl [data not shown]). (d-k) Delta/Notch-deficient mindbomb mutants (mib-/-) lack MFP cells (f) and were identified by an increase in isl1-positive neurons (ne, in red [g,i,k]). (h-k) Mdka overexpression and knockdown produces identical MFP and notochord phenotypes in mib-/- as in wild-type embryos. Overexpression enlarges the MFP (n = 11/15, 73% [h,i]) and knockdown results in MFP gaps larger than in noninjected mib-/- (n = 11/18, 61% [j]). (l-n) one-eyed pinhead (oep-/-) embryos that lack the entire MFP (m) were rescued by injection of mdka RNA (n = 12/14, 86% [n]). In these embryos, the broadness of the MFP was increased. oep-/- were identified by remaining gaps in the f-spondin-positive MFP (arrowhead). (o,p) Overexpression of cyclops (cyc) RNA in wild-type embryos resulted in an enlargement of the MFP from one to three cells in width (n = 36/57, 63% [o]). Coinjection of cyc RNA and mdka morpholino resulted in a MFP with nearly wild-type morphology (n = 73/87, 83% [p]). (q-v) In no-tail mutants (ntl-/-), MFP width is enlarged to variable extents (q,r,u), but is nearly rescued after mdka morpholino injection (s,v). ntl-/- embryos were identified by the absence of a notochord and oep expression (q-s,u,v). mdka knock-down is not able to rescue the notochord. Anterior is to the left in lateral (a-c,d,f,h,j) and dorsal (l-s) views; arrowheads indicate gaps in MFP. (e,g,i,k,t-v) Transverse sections at trunk level. Bars: c, 100 µm; p,s, 40 µm; j,k,t, 20 µm. EXPRESSION / LABELING:
|
|
mdka transcription is not regulated by early acting floor plate-inducing factors. (a,b) In mib-/- embryos, identified by an enlarged notochord and an altered somite shape, mdka expression is normal compared with wild-type embryos (n = 14). (c,e,g) ntl, oep, and flh are expressed in the notochord of the trunk and progenitor cells in the tailbud. (c) In addition, ntl is also expressed in the posterior tailbud region. (d) In ntl-/- mutants that lack a notochord and have an enlarged MFP, mdka expression is not impaired (n = 24). In these embryos, the paraxial mesoderm is separated at the midline by an expanded MFP. (f) oep-/- mutants identified by their characteristic head phenotype form a normal notochord, but lack MFP. In oep-/-, mdka expression is normal (n = 11). (h) In flh-/- mutants, mdka is expressed at the midline (n = 25). (i) Two-phase model for MFP formation during neurulation in the zebrafish embryo. Midline precursor cells (MPC) in the tailbud are initially induced to adopt MFP fate in a first Mdka-independent phase. As the embryo extends caudally, Mdka derived from the trunk paraxial mesoderm (pm) regulates allocation of MFP cells (second phase). All pictures are dorsal views of the posterior trunk and tail; anterior is to the top. Arrowheads mark the position of the posterior front of mdka expression in the paraxial mesoderm. Bar: h, 100 µm.
EXPRESSION / LABELING:
|
|
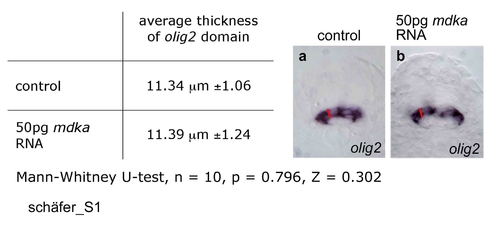
Effect of mdka overexpression on the size of the olig2 motoneuron progenitor domain. The dorsoventral width of the olig2 domain was determined in transverse sections of control embryos (a) and embryos injected with 50pg mdka RNA (b) using the SIS image analysis software. Seven sections at different anteroposterior trunk levels of 10 embryos each were analysed. No significant difference in average width was observed (Mann-Whitney U-test, n = 10, p = 0.796, Z = 0.302). EXPRESSION / LABELING:
|
|
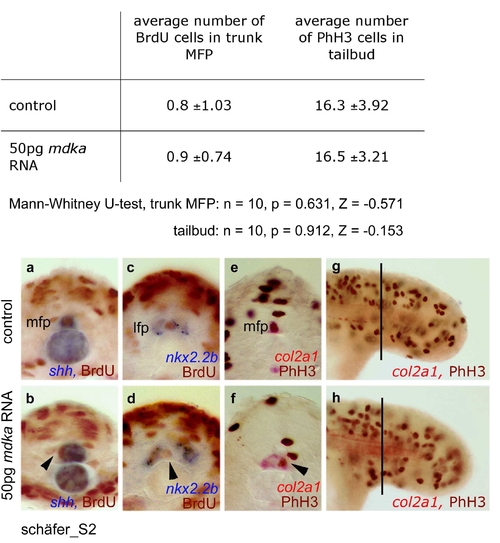
Effect of mdka overexpression on proliferation in MFP and tailbud. In the shh expressing MFP, BrdU positive cells in S phase were counted in ten transverse trunk sections each of ten control embryos (a) and ten embryos injected with 50pg mdka RNA (b). No significant changes in cell numbers were observed (Mann-Whitney U-test, n = 10, p = 0.631, Z = -0.571). This was also confirmed in independent BrdU analyses using nkx2.2b as LFP marker (n = 5; c,d). Identical results were obtained when MFP cells in M phase were counted after immunostaining with a phospho-histone H3 (PhH3) antibody and col2a1 as MFP marker (n = 5; e,f). Also in the tailbud, the number of M phase cells was not significantly increased (Mann-Whitney U-test, n = 10, p = 0.912, Z = -0.153; g,h). For counting cells in the tailbud, one focal plane (focused on MFP) of a defined trunk area (with respect to somite positions) was used. Arrowheads indicate BrdU or PhH3 positive cells in the MFP. For BrdU labelling, embryos were incubated for 30 minutes in 10 mM BrdU (Sigma) at the 18s stage and fixed in 4% paraformaldehyde after washing for 15 minutes. Incorporated BrdU, as well as PhH3 protein were detected after shh, col2a1 or nkx2.2b in-situ hybridization by immunostaining with the respective antibodies (anti-BrdU G3G4, Developmental Studies Hybridoma Bank, University of Iowa; anti-PhH3, Upstate Biotechnology, Lake Placid, NY) and the Vecta Stain Elite Kit (Vector Laboratories, Burlingame, CA). EXPRESSION / LABELING:
|
|
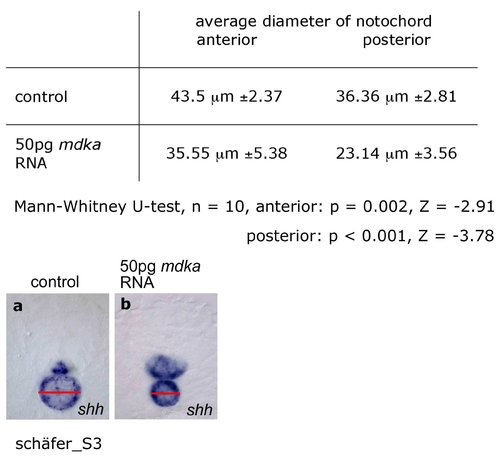
Effect of mdka overexpression on notochord size. The diameter of the notochord was determined (SIS imaging analysis software) in six transversial sections (three at anterior, three at posterior trunk levels at equivalent positions with respect to somite positions) each of ten control embryos (a) and ten embryos injected with 50pg mdka RNA (b) after staining for shh. A highly significant reduction of notochord diameter was observed in anterior (Mann-Whitney U-test, n = 10, p = 0.002, Z = -2.91) and posterior trunk (Mann-Whitney U-test, n = 10, p < 0.001, Z = -3.779). EXPRESSION / LABELING:
|
|
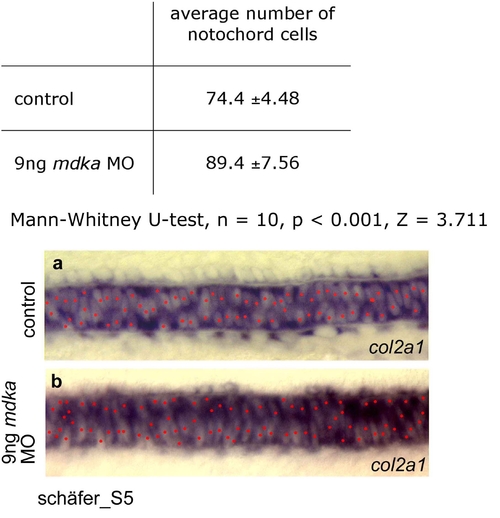
Effect of mdka knock-down on notochord cell number. Notochord cells were counted in defined regions of the medial trunk (with respect to somite positions) of ten control embryos (a) and ten embryos injected with 9ng mdka morpholino (b). A highly significant increase in cell number was observed in morpholino injected embryos (Mann-Whitney U-test, n = 10, p < 0.001, Z = 3.711). Notochord cells were visualized by col2a1 expression and nuclear regions devoid of in-situ staining were marked (in red). For manual counting of notochord cells in whole mount preparations, one focal plane (focused on the MFP) was analyzed. EXPRESSION / LABELING:
|

Unillustrated author statements EXPRESSION / LABELING:
|