- Title
-
The hedgehog-PKA pathway regulates two distinct steps of the differentiation of retinal ganglion cells: the cell-cycle exit of retinoblasts and their neuronal maturation
- Authors
- Masai, I., Yamaguchi, M., Tonou-Fujimori, N., Komori, A., and Okamoto, H.
- Source
- Full text @ Development
|
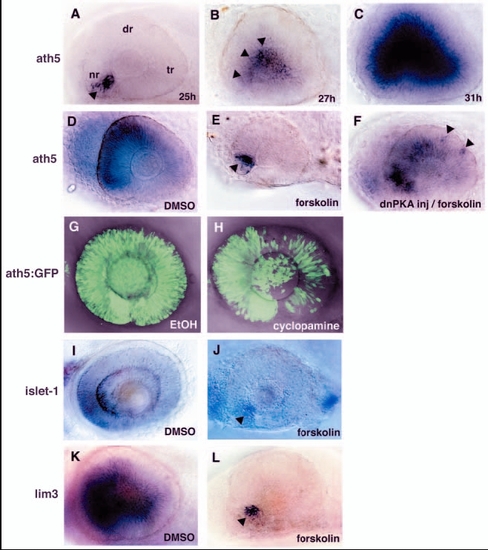
PKA inhibits the wave of neurogenesis in the zebrafish retina. (A-C) In situ hybridisation of embryos at 25 (A), 27 (B) and 31 (C) hpf with an ath5 RNA probe. ath5 mRNA expression is initiated at the ventronasal retina (A, arrowhead) and progresses to the central region of the neural retina (B, arrowheads). (D,E) ath5 expression in the 33-hpf retina treated with forskolin (E) or with DMSO as a control (D). In the forskolin-treated retina, ath5 expression is initiated normally in a few cells adjacent to the optic stalk tissue (E, arrowhead), but does not spread to the dorsal and temporal retina. (F) ath5 expression in the 33-hpf forskolin-treated embryo in which dnPKA is overexpressed. Overexpression of dnPKA rescues ath5 expression. Note that ath5 expression is observed in isolated cells in the dorsotemporal retina (arrowheads), suggesting cell-autonomous rescue of ath5 expression. (G,H) ath5:GFP expression in 48-hpf cyclopamine-treated (H) and control (G) retinas. The progression of ath5:GFP expression is mildly delayed in the presence of cyclopamine. (I-L) In situ hybridisation of forskolin-treated and control retinas with islet1 (I,J) and lim3 RNA probes (K,L). Lateral view of optic cups at 48 (I,J) and 33 (K,L) hpf. Both islet1 and lim3 are expressed in a few cells adjacent to the optic stalk (arrowheads), but their expression does not progress in the presence of forskolin (J,L). nr, nasal retina; dr, dorsal retina; tr, temporal retina. |
|
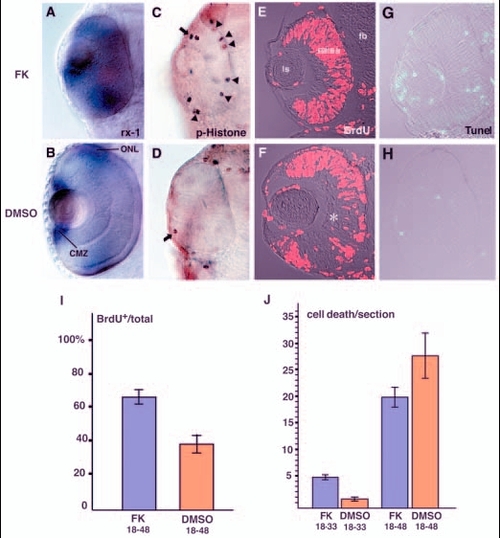
PKA inhibits cell-cycle exit of retinal progenitor cells. (A,B) rx1 expression in 48-hpf forskolin-treated (A) and DMSO-treated (B) retinas. rx1 is expressed both in the outer nuclear layer (ONL) and in the CMZ of DMSO-treated retinas (B); expression is not downregulated in the presence of forskolin (A). (C,D) Labelling of 33-hpf retinas with anti-phosphorylated histone H3 antibody. In the forskolin-treated retina (C), antibody staining was observed in the CMZ (arrow) and at the ventricular surface of the neural retina (arrowheads), whereas only a few mitotic cells were observed in the CMZ (arrow) of the DMSO-treated retina (D). (E,F) BrdU labelling of 48-hpf forskolin-treated (E) and DMSO-treated (F) retinas. In the forskolin-treated retina, most of retinal cells are BrdU positive, whereas cell division seems to cease in the RGC and amacrine cell layers (asterisk) in the DMSO-treated retina. (G,H) TUNEL analysis of 33-hpf forskolin-treated (G) and DMSO-treated (H) retinas. Patterns of cell apoptosis (green) do not correlate with those of neuronal production in forskolin-treated retinas (G; data not shown), although the number of apoptotic cells is higher than in DMSO-treated retinas (H). (I) Ratios of BrdU-positive areas to total area in forskolin- and DMSO-treated retinas. Bars indicate the average ratios that were observed in cryosections of the retinas labelled with the anti-BrdU antibody. The numbers of embryos examined were n=3 and n=2 for forskolin treatment (FK 18-48) and DMSO treatment (DMSO 18-48) between 18 and 48 hpf, respectively. Four sections from the nasal to temporal regions were examined per embryo. (J) Quantification of apoptotic cells in forskolin- and DMSO-treated retinas. Bars indicate the average number of apoptotic cells observed in cryosections corresponding to the central retina subjected to TUNEL. One section was examined per embryo. The numbers of embryos examined were n=7 for forskolin treatment between 18 and 33 hpf (FK 18-33), n=3 for DMSO treatment between 18 and 33 hpf (DMSO 18-33), n=5 for forskolin between 18 and 48 hpf (FK 18-48), and n=3 for DMSO treatment between 18 and 48 hpf (DMSO 18-48). CMZ, ciliary marginal zone; fb, forebrain; FK, forskolin; ls, lens; ONL, outer nuclear layer. EXPRESSION / LABELING:
|
|
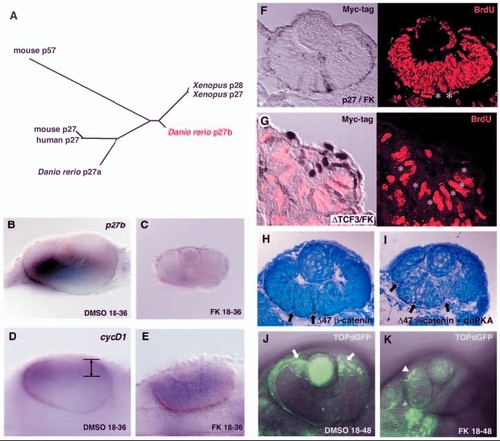
Canonical Wnt signalling is required for PKA-mediated cell proliferation. (A) Phylogenetic relationship among vertebrate p27 proteins and mouse p57. The length of each branch is proportional to sequence divergence from the branch points. (B,C) In situ hybridisation of 36-hpf DMSO- and forskolin-treated retinas with a p27b RNA probe. p27b is expressed in the DMSO-treated retina (B) but not in the forskolin-treated retina (C). (D,E) In situ hybridisation of 36-hpf DMSO-treated (D) and forskolin-treated (E) retinas with a cyclin D1 RNA probe. cyclin D1 is downregulated in differentiating neurons and is localised in the CMZ of the DMSO-treated retina (D, lines/arrows). However, cyclin D1 is not downregulated in the forskolin-treated retina (E) but remains expressed in a large region. (F) 48-hpf forskolin-treated embryos expressing myc-tagged p27 labelled with the anti-myc antibody (brown in left panel) and anti-BrdU antibody (red in right panel). Retinal cells expressing p27 are BrdU negative (white asterisks), whereas almost all p27-negative cells incorporate BrdU. (G) 33-hpf forskolin-treated embryos expressing myc-tagged N-Tcf3 labelled with the anti-myc antibody (brown in left panel) and anti-BrdU antibody (red). Retinal cells expressing ΔN-Tcf3 are BrdU negative (white asterisks). (H,I) 48-hpf wild-type retinas expressing Δ47-ß-catenin (H) and a mixture of Δ47-ß-catenin and dnPKA (I). Multi-folded neural retina is observed (arrows) in both cases, suggesting that dnPKA does not inhibit Wnt-induced proliferation. (J,K) GFP expression in 2-dpf TOPdGFP transgenic retinas treated with DMSO (J) and forskolin (K). GFP is expressed in the CMZ of the DMSO-treated retina (J, arrows and white dashed lines). In the forskolin-treated retina, GFP expression is not detected. Arrowhead (K) indicates GFP expression in epidermis. EXPRESSION / LABELING:
|
|
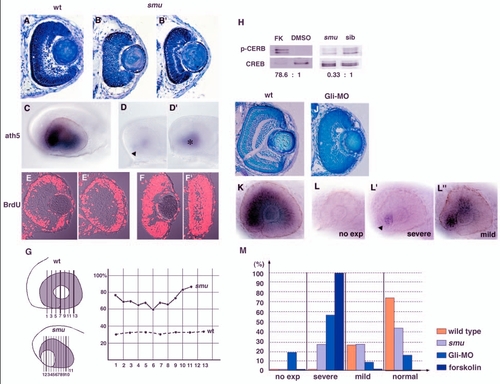
The wave of retinal neurogenesis is affected in smu mutant and Gli-MO-injected embryos. (A-B′) Plastic sectioning of wild-type (A) and smub577?/? (B,B′) retinas at 48 hpf. In smub577?/? retinas, lamination defects vary. No lamination is observed in the most severe phenotype (B) and a nearly normal lamination occurs in the mildest phenotype (B′). (C-D′) ath5 expression in wild-type (C) and smub577?/? (D,D′) retinas at 33 hpf. ath5 expression is initiated at the ventro-nasal retina (D, arrowhead) but does not spread to the entire eyes in smub577?/? embryos. This phenotype varies from severe (D) to mild (D′, asterisk), and the defect in ath5 expression is proportional to that of a cyclopic phenotype. (E-F′) Labelling of 48-hpf wild-type (E,E′) and smub577?/?(F,F′) retinas with the anti-BrdU antibody. (E,F) Central retinas of wild-type (E) and smub577?/? embryos (F). These sections correspond to no.7 (wild-type) and no.2 (smu?/?) shown in G, respectively. (E′,F′) Peripheral retinas of wild-type (E′) and smub577?/? (F′) embryos. These sections correspond to no.13 (wild-type) and no.10 (smu?/?) shown in G, respectively. (G) Spatial profile of the ratio of BrdU-positive area to total area in wild-type and smub577?/? retinas. Solid and dotted lines indicate smu?/? and wild-type sibling retinas, respectively. Numbers represent anterior-posterior locations of cryosections, which are shown in the left schematic drawing. (H) Western blot analysis of forskolin-treated and smub577?/? heads using antibodies against Ser133-phosphorylated CREB (upper) and CREB (lower). The phosphorylation of CREB is more than 70 times higher in forskolin-treated heads than in control DMSO-treated heads. However, in smub577?/? heads, CREB phosphorylation is decreased to one-third of the normal level. (I,J) Plastic sectioning of wild-type (I) and Gli-MO-injected (J) retinas at 3 dpf. In the severe case of Gli-MO-injected embryos, retinal lamination is severely delayed. (K-L′′) In situ hybridisation of 33-hpf wild-type (K) and Gli-MO-injected (L-L′′) embryos with an ath5 RNA probe. The progression of ath5 expression is perturbed to different degrees in Gli-MO-injected retinas, from no initiation (L), to severe (L′) or mild (L′′) inhibition. In the severe case, ath5 expression is only observed in the ventronasal retina (L′, arrowhead). (M) Quantitative assessment of ath5 expression in wild-type (orange), smu mutant (light blue), Gli-MO-injected (blue) and forskolin-treated (dark blue) embryos. Embryos were classified into four groups according to the severity of the defect in ath5 expression, as shown in (L-L′′) and counted in number. Numbers of examined embryos were n=11 for wild type, n=21 for the smu mutant, n=63 for Gli-MO-injected embryos and n>100 for forskolin-treated embryos. Almost all of the forskolin-treated embryos show the `severe′ phenotype. FK, forskolin. EXPRESSION / LABELING:
|
|
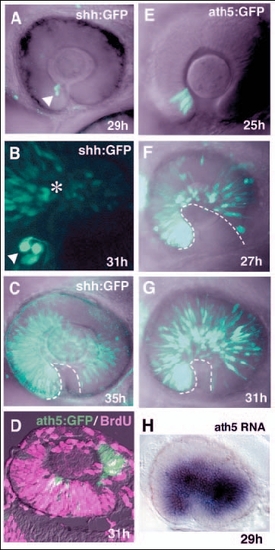
Pattern of progression of shh:GFP and ath5:GFP expression in zebrafish retina. (A-C) Lateral view of the optic cup of the transgenic line Tg(shh:GFP). B is shown at twice the magnification of A and C. shh:GFP is expressed in a few cells adjacent to the optic stalk at 29 hpf (A, white arrowhead). Progression of GFP expression is observed in the adjacent dorsal region at 31 hpf (B, asterisk). A wave front of shh:GFP expression reaches the temporal region of the neural retina at 35 hpf (C, dotted line). (D) Double labelling of 31-hpf retinas of the transgenic line Tg(ath5:GFP) with the anti-BrdU antibody and ath5:GFP antibody. ath5:GFP-positive cells (green) are BrdU (magenta) negative. (E-G) GFP expression in the transgenic line Tg(ath5:GFP). Throughout the stages examined, the pattern of ath5:GFP expression is similar to that of ath5 transcription. Note that GFP-expressing cells are already observed in the temporal region at 27 hpf (F), and the density of GFP-positive cells increases in later stages (G). The dotted line indicates a wave front of ath5:GFP expression. (H) ath5 RNA expression in the transgenic line Tg(shh:GFP) at 29 hpf. This is the same embryo that is shown in A. ath5 expression spreads to the large region of the neural retina. |
|
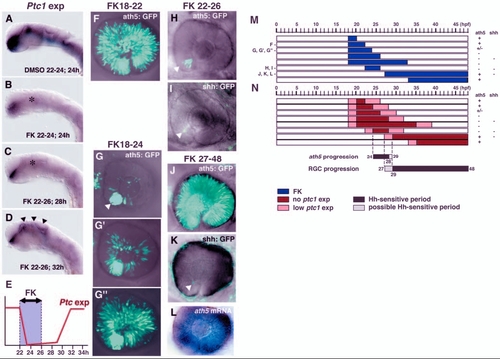
Hh signalling regulates two distinct steps of RGC differentiation. (A,B) In situ hybridization of 24-hpf embryos with a ptc1 RNA probe. Embryos were treated with DMSO (A) and forskolin (B) from 22 to 24 hpf. ptc1 expression is observed in the ventral CNS in DMSO-treated embryos (A), but is drastically reduced in forskolin-treated embryos (B, asterisk), although weak expression is still observed in the ventral forebrain. (C,D) ptc1 expression of embryos treated with forskolin between 22 and 26 hpf. ptc1 expression is still low at 28 hpf (C, asterisk), but has recovered at 32 hpf (D, arrowheads). (E) Schematic diagram of the time lag between forskolin treatment and inhibition of the Hh signalling pathway. ptc1 expression is inhibited within 2 hours of the start of forskolin treatment. ptc1 expression is still low 2 hours after the removal of forskolin, but has recovered within 6 hours of the removal of forskolin. (F) ath5:GFP expression in retina treated with forskolin from 18 to 22 hpf; ath5:GFP expression progresses throughout the neural retina. (G-G′′) ath5:GFP expression in retinas treated with forskolin from 18 to 24 hpf. The inhibition of progression varies from severe (G), to mild (G′) and nearly normal (G′′). In the severe case, ath5:GFP is observed only in the ventronasal retina (white arrowhead). (H) ath5:GFP expression in retina treated with forskolin from 22 to 26 hpf. The progression of ath5:GFP expression is completely blocked (white arrowhead). (I) shh:GFP expression in retina treated with forskolin from 22 to 26 hpf. shh:GFP expression also fails to progress from the ventronasal retina (white arrowhead). (J) ath5:GFP expression in retina treated with forskolin from 27 to 48 hpf. The propagation of ath5:GFP expression occurs normally throughout the neural retina. (K) shh:GFP expression in retina treated with forskolin from 27 to 48 hpf. The progression of shh:GFP expression remains blocked (white arrowhead). Weak background signals are observed in the skin surrounding the eye. (L) ath5 RNA expression in the same embryo as shown in K. ath5 RNA expression spreads to whole region of the neural retina. (M) Pulse treatment with forskolin in different time windows. Blue bars indicate the period of treatment with forskolin. All embryos were examined at 48 hpf. +, progression of ath5:GFP or shh:GFP expression occurs normally; ? indicates that progression is effectively blocked. (N) Time-window of Hh signalling inhibition, estimated by considering the time lag between forskolin treatment and the downregulation of ptc1 expression. Red bars indicate the period when ptc1 expression is severely suppressed by forskolin treatment. Pink bars indicate the time lag between the start of forskolin treatment and the suppression of ptc1 expression, or between the removal of forskolin and the recovery of ptc1 expression; within these time periods Hh signalling may be reduced. Black and grey bars indicate Hh-sensitive and possible Hh-sensitive periods for the progression of ath5 expression and RGC maturation, respectively. FK, forskolin. |
|
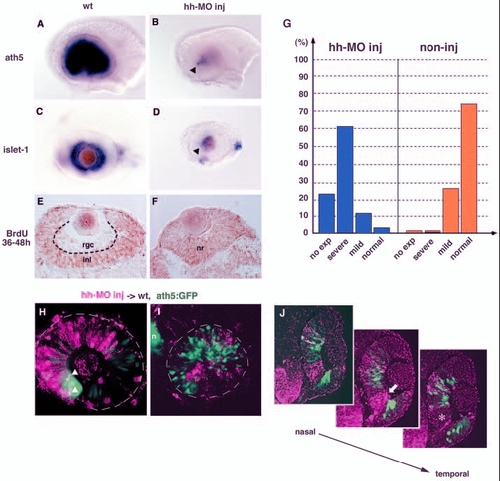
Shh and Twhh regulate the progression of ath5 expression. (A,B) ath5 expression in 33-hpf wild-type (A) and Hh-MO-injected (B) retinas. ath5 expression fails to progress from the ventronasal retina injected with Hh-MO (arrowhead). (C,D) islet1 expression in 48-hpf wild-type (C) and Hh-MO-injected (D) retinas. islet1 expression fails to progress from the ventronasal retina injected with Hh-MO (arrowhead). (E,F) Labelling of 48-hpf wild-type (E) and Hh-MO injected (F) retinas with an anti-BrdU antibody (brown). Because BrdU is incorporated from 36 to 48 hpf, the RGC layer is BrdU negative in the wild-type retina. By contrast, almost all retinal cells are labelled with the anti-BrdU antibody in the Hh-MO-injected retina. (G) Quantitative assessment of ath5 expression in Hh-MO-injected (blue) and non-injected control (orange) embryos. The defect in ath5 expression in Hh-MO-injected embryos shows a similar profile to that in Gli-MO-injected embryos (blue bars in Fig. 4M). Numbers of Hh-MO-injected and non-injected embryos examined were 33 and 11, respectively. (H) Hh-MO-injected cells were incorporated into 36-hpf wild-type peripheral retina. ath5:GFP expression (green) does not progress towards the Hh-MO-positive area (magenta), although double-positive cells (white arrowheads) are observed at the interface between wild-type and Hh-MO areas. (I) Hh-MO-injected cells were incorporated into 36 hpf wild-type central retina. Hh-MO (magenta) and ath5:GFP (green) are segregated. (J) Three adjacent sections are shown along the axis from the nasal to temporal regions. ath5:GFP expression (green) does not occur in retinal columns derived from Hh-MO-injected donor embryos (magenta). GFP is not expressed even in wild-type retinal columns (white asterisk in right panel) located adjacent to the temporal side of Hh-MO-derived retinal columns (white arrow in middle panel). inl, inner nuclear layer; n, nose; nr, neural retina; rgc, retinal ganglion cell layer. |