Figure 3—figure supplement 4—source data 1.
- ID
- ZDB-FIG-241218-39
- Publication
- Sun et al., 2024 - Ciliary length regulation by intraflagellar transport in zebrafish
- Other Figures
-
- Figure 1.
- Figure 2
- Figure 2—figure supplement 1.
- Figure 3
- Figure 3—figure supplement 1—source data 1.
- Figure 3—figure supplement 2—source data 1.
- Figure 3—figure supplement 3—source data 1.
- Figure 3—figure supplement 4—source data 1.
- Figure 4
- Figure 4—figure supplement 1—source data 1.
- Figure 4—figure supplement 2—source data 1.
- Figure 4—figure supplement 3—source data 2.
- Figure 4—figure supplement 4—source data 1.
- Figure 5
- All Figure Page
- Back to All Figure Page
|
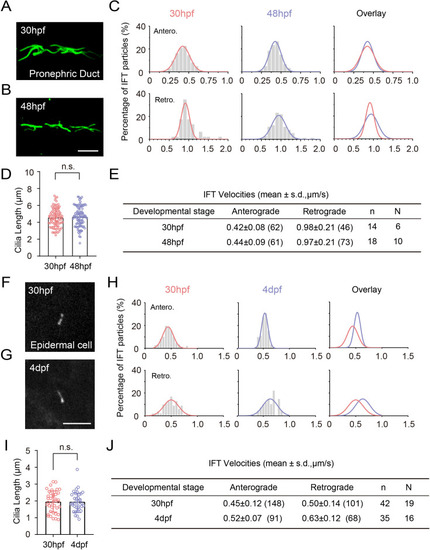
Comparison of cilia length and IFT volecity at different developmental stages. ( |