- Title
-
Zebrafish narrowminded suggests a genetic link between formation of neural crest and primary sensory neurons
- Authors
- Artinger, K.B., Chitnis, A.B., Mercola, M., and Driever, W.
- Source
- Full text @ Development
|
Phenotypic basis for isolation of nrd during the genetic screen. Dorsal views of 2-3 somite stage haploid embryos (anterior to the right) upon analysis by in situ hybridization utilizing a combination of RNA probes: her5 (to visualize the midbrain hindbrain boundary), Krox20 (to visualize rhombomeres 3 and 5) and HuC (to visualize trigeminal ganglia (TG) and medial (M), intermediate (I) and lateral (L) domains of primary neurons). Wild type (A,C) and nrd (B,D) embryos with view of anterior (A,B) and trunk neural plate (C,D) region. nrd has normal anterior-posterior patterning but lacks the most lateral stripe of primary neurons. EXPRESSION / LABELING:
|
|
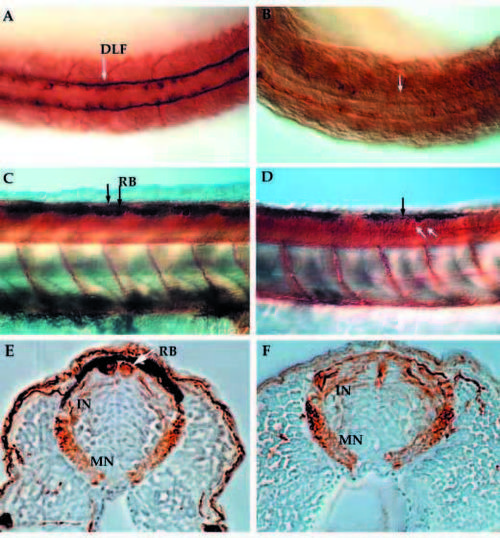
Anti-acetylated α-tubulin immunostained whole-mount embryos at 24 hpf (A,B) and 48 hpf (C-F) reveal the neuronal pattern in nrd. Dorsal view of the neuronal pattern of a 24 hpf wild-type embryo (A) shows large RB neurons (arrow; large brown cells) as well as a distinct DLF (two parallel tracts of axons). In nrd (B), the embryos have very little dorsal expression of a-tubulin, except in a few scattered commissural neurons (dark brown stained cells). At 48 hpf, the differentiation of many neuronal cell types including large RB neurons can be seen in wild-type embryos (C, arrows and lateral view). In nrd (D), other neurons such as commissural neurons appear to develop normally (white arrows), but RB neurons do not form (black arrow point to area where RBs should form). (E,F) Transverse sections of embryos in C and D (embryos were embedded in plastic and sectioned at a thickness of 3 mm). In wild-type embryos, RBs are large neurons at the dorsal most aspect of the neural tube (E), while nrd embryos lack RBs. RB, Rohon Beard cell; DLF, dorsal longitudinal fascicle; In, interneuron; MN, motorneuron. |
|
Visible phenotype of nrd and defects in formation of neural crest derivatives. (A) Whole live nrd (top) and wild-type sibling (bottom) at 48 hpf (lateral view). Wild-type (B,D,F) and nrd (C,E,G) siblings (10x). nrd mutant larvae have a smaller number of pigment cells (B,C: dorsal view), reduced fin mesenchyme (D,E: lateral view) and a normal pattern of cartilage (F,G: ventral view, visualized in whole mount by alcian blue stain. The slight reduction of density of the stain is not observed in serial sections). PHENOTYPE:
|
|
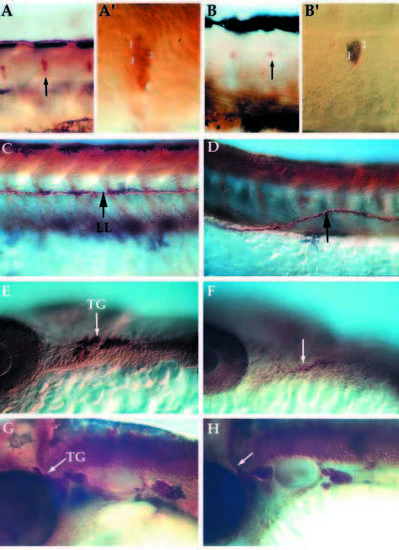
Lateral views of whole mount embryos immunostained for expression of acetylated α-tubulin and anti-HuC in neural crest derivatives at 24-72 hpf. Pattern of DRG, lateral line pathfinding, trigeminal ganglia formation in wild-type (A,C, trunk; E,G, head) and nrd mutant embryos (B,D, trunk; F,K, head). nrd embryos have a reduction in the number of neurons per ganglion in the DRG at 72 hpf (B; see also Table 1) and the neuromasts (not shown) (A′,B′) High magnification images of the ganglion (arrows in A and B) to show the reduction in the number of cells per ganglion. (C,D) The lateral line (LL) pathfinding is misaligned (arrow) at 72 hpf. Within the trigeminal ganglia (TG), cells derive from the neural crest as well as ectodermal placode. At 24 hpf (E,F), mutant embryos (F) show a reduction in the dorsal ganglia corresponding to the neural crest derived portion (arrows). But by 48 hpf (G,H), the trigeminal ganglia in the mutant (H) is close to the normal size, suggesting that the placodal or late migrating neural crest population may be compensating for the loss of the neural crest. DRG, dorsal root ganglia. PHENOTYPE:
|
|
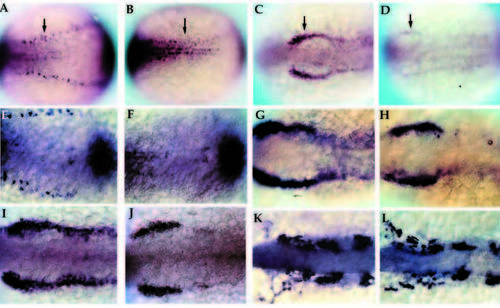
Early development of primary neurons and neural crest in nrd. Wholemount in situ hybridization of 2-3 somite stage zebrafish embryos with HuC (A,B; 20x), snail2 at the 2 somite stage (C,D; 20x), BMP-4 (E,F; 32x), snail2 at the 4-5 somite stage (G,H; 32x), fkd6 at the 2-3 somite stage (I,J; 32x), and dlx2 at the 10 somite stage (K,L; 32x). All images show dorsal views of embryos orientated anterior to the left. Wildtype (A,C,E,G,I,K) and nrd mutant (B,D,F,H,J,L) embryos. nrd embryos shows a lack of HuC expression in the lateral most stripe of primary neurons, corresponding to the lateral edge of the neural plate (arrows in A,B). snail2 expression indicates a severe reduction in the neural crest population in the mutant embryos (arrows in C,D). This is also consistant with effects on the neural crest expression domain of fkd6, which appears reduced in nrd although less severe than snail2 (I,J). BMP-4 expression appears normal in nrd (compare E and F; double stain with HuC to identify mutant embryos). Later in development, at the 4-5 somite stage, snail2 expression appears to be upregulated again (compare nrd, in H, to wild type, in G) and is almost indistinguishable from wild type at the 10 somite stage, shown here with dlx2 expression (K,L). EXPRESSION / LABELING:
|
|
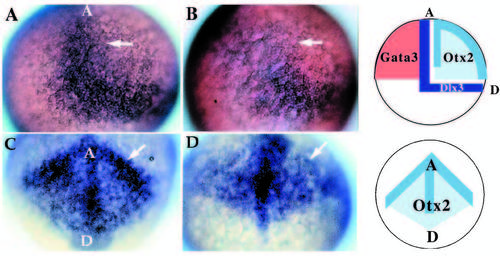
Patterning abnormalities in the early neural plate. Expression of genes involved in patterning the early neural plate visualized by whole-mount in situ hybridization for dlx3 (A,B; 20x) and otx2 (C,D; 20x) at late gastrulation stages (90% epiboly). Wild-type (A) and mutant (B) embryos indicate the lack of the anterior-medial domain of dlx3 (arrow) in nrd. In these embryos, anterior is to the top, dorsal to the right. (C,D) Anterior view of otx2 expression in wild type (C) and nrd (D): at the border region of the neural plate otx2 expression level is reduced (arrow) but appears to extend in a pattern of normal shape in mutant embryos. In C and D, the anterior edge of the neural plate is to the top, with a dorsal and posterior neural plate toward the bottom. Schematic diagrams illustrate the normal expression domains of dlx3 in the neural crest and placodal domain, between the neural and nonneural ectoderm, otx2 in the neural plate, and GATA3 in the non-neural ectoderm in wild-type embryos. GATA3 expression is not affected in nrd embryos (not shown). EXPRESSION / LABELING:
|
|
DV patterning is normal in nrd. Expression analysis of the mesodermal genes BMP-4, Snail1, Brachyury and SCL in wild-type (A,C,E,G) and nrd (B,D,F,H) embryos at 80-90% epiboly to 5 somite stage. (A,B) BMP-4 is expressed on the ventral side of the gastrula and the leading edge of the invagingating tissue on the dorsal side in both wild-type and nrd embryos. Anterior top and dorsal to the right. (C,D) Dorsal view of the expression of snail1 as seen by in situ hybridization shows the normal paraxial mesoderm in nrd (anterior to the top). (E,F) Zebrafish Brachyury is expressed in a ring around the blastopore and in the forming notochord at the end of gastrulation. nrd embryos show a normal pattern of expression of Brachyury (F) as compared to wild type (E). Dorsal view, anterior to the top in both E and F. (G,H) Expression of SCl in the blood precursors at the 5 somite stage shows normal development of a mesoderm derivative, blood, at a slightly later stage in an nrd (H) embryo compared to a wild type (G). This region is just ventral to the neural crest forming region. (Note: nrd embryos presented in this figure were from nrd mutant clutches in which homozygous were not distinguishable from heterozygous siblings) D, dorsal; A, anterior; P, posterior. EXPRESSION / LABELING:
|
|
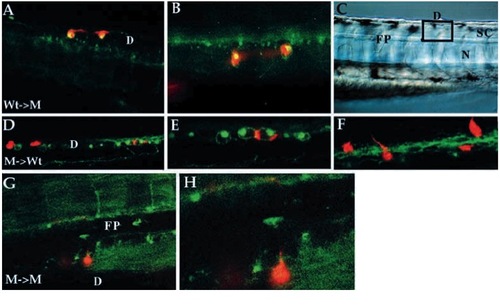
Cell-autonomous action of nrd as revealed by mosaic analysis. Results of cell transplantation experiments between wild-type and nrd embryos viewed by confocal microscopy (A,B,D-H; all images are anterior to the left, dorsal up. Some images were flipped horizontal to maintain consistant antero-posterior orientation.). Low (A) and high (B) magnification of wild-type cells (LRD-labeled red) transplanted into a mutant environment that are able to differentiate into RB neurons (as revealed by HNK-1 antibody staining, green; where HNK-1 epitope expression is present in the same cell that contains the lineage label, the cells appear yellow). (C) Nomarski image of a similar live embryo to illustrate the location of the labelled cells in the dorsal neural tube (square indicates approximate location of confocal optical section for all embryos shown). Mutant cells (D-F, red) transplanted into a wild-type environment have never been observed to be able to form RB neurons although they can form other neuronal cell types in or near the RB forming domain of the neural cord. (E,F) Higher magnification views of two different optical planes showing that mutant cells (red) located within the wild-type domain of HNK-1-expressing cells are able to form commissural neurons (see axonal processes in F). Low and high magnification view of a mutant cell (G,H; red) transplanted into a mutant environment that forms commissural neurons with correct pathfinding ability. This axon can be seen crossing the floorplate on the contralateral side and projecting along the DLF. D, dorsal spinal cord; FP, floor plate; SC, spinal cord; N, notochord. |