- Title
-
RFC2 may contribute to the pathogenicity of Williams syndrome revealed in a zebrafish model
- Authors
- Park, J.W., Choi, T.I., Kim, T.Y., Lee, Y.R., Don, D.W., George-Abraham, J.K., Robak, L.A., Trandafir, C.C., Liu, P., Rosenfeld, J.A., Kim, T.H., Petit, F., Kim, Y.M., Cheon, C.K., Lee, Y., Kim, C.H.
- Source
- Full text @ J. Genet. Genomics
|
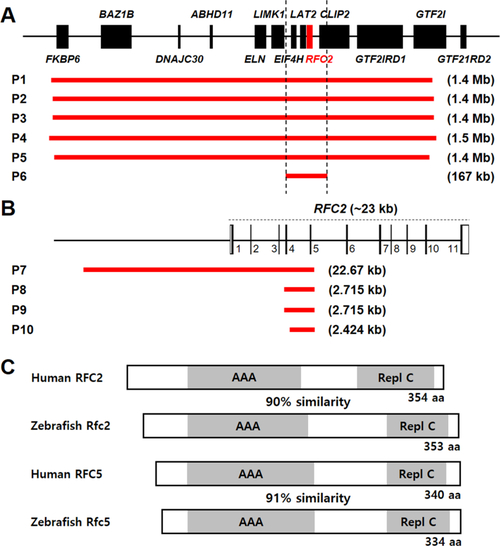
WS patients with 7q11.23 microdeletions and |
|
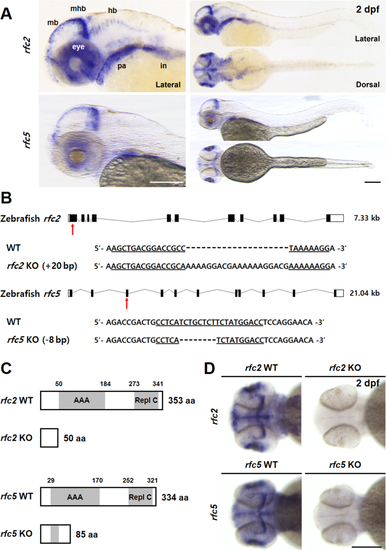
Generation of EXPRESSION / LABELING:
|
|
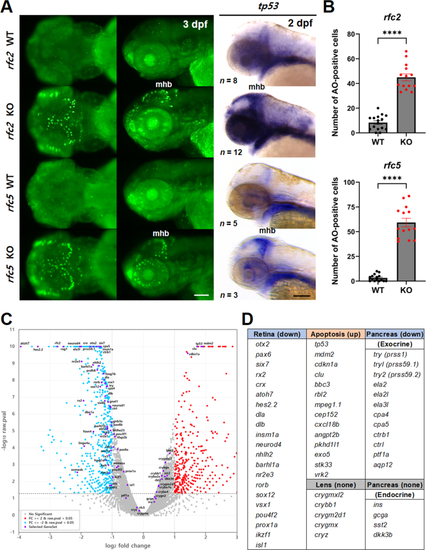
Reduced head and brain size in |
|
Increased cell death in EXPRESSION / LABELING:
PHENOTYPE:
|
|
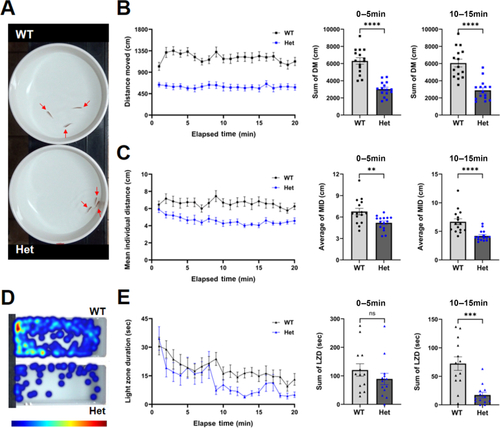
Skeletal and vascular phenotypes in |
|
Increased social cohesion and anxiety-like behavior in Het adult PHENOTYPE:
|

Unillustrated author statements PHENOTYPE:
|
Reprinted from Journal of genetics and genomics = Yi chuan xue bao, 51(12), Park, J.W., Choi, T.I., Kim, T.Y., Lee, Y.R., Don, D.W., George-Abraham, J.K., Robak, L.A., Trandafir, C.C., Liu, P., Rosenfeld, J.A., Kim, T.H., Petit, F., Kim, Y.M., Cheon, C.K., Lee, Y., Kim, C.H., RFC2 may contribute to the pathogenicity of Williams syndrome revealed in a zebrafish model, 1389-1403, Copyright (2024) with permission from Elsevier. Full text @ J. Genet. Genomics