- Title
-
Deoxyhypusine synthase deficiency syndrome zebrafish model: aberrant morphology, epileptiform activity, and reduced arborization of inhibitory interneurons
- Authors
- Shojaeinia, E., Mastracci, T.L., Soliman, R., Devinsky, O., Esguerra, C.V., Crawford, A.D.
- Source
- Full text @ Mol. Brain
|
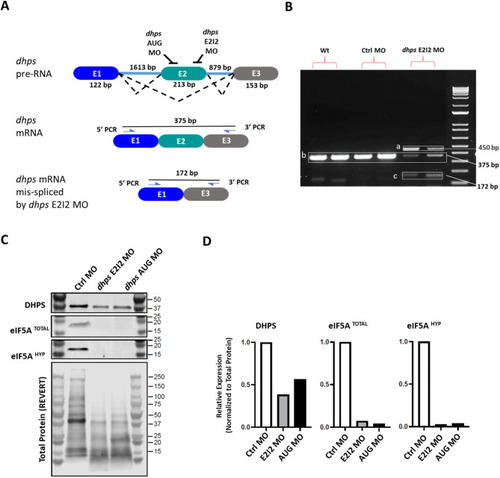
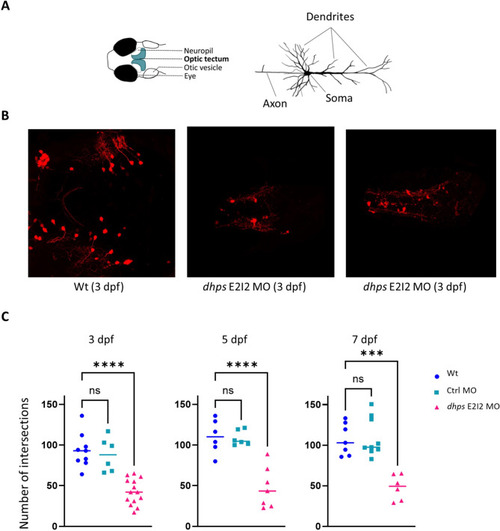
Generation of the DHPS deficiency zebrafish model. ( |
|
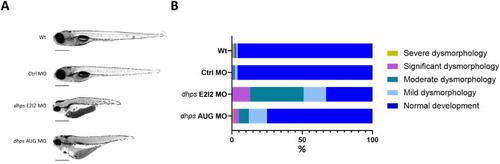
Phenotypic analysis of PHENOTYPE:
|
|
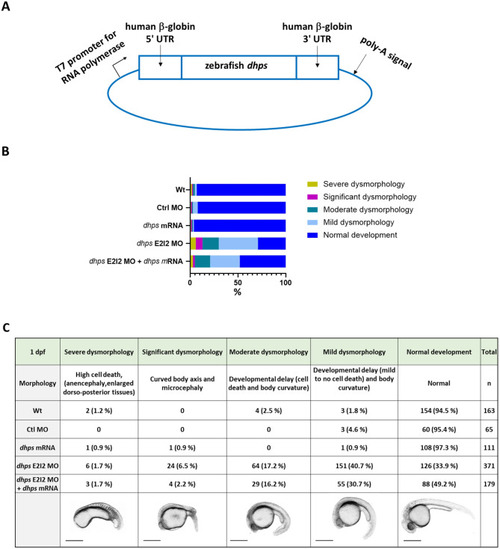
Partial rescue of PHENOTYPE:
|
|
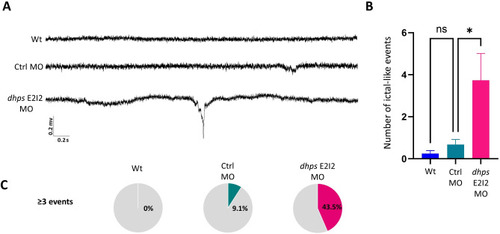
LFP recording of PHENOTYPE:
|
|
GABAergic neuronal dendritic arborization. ( |