- Title
-
Foxd1-dependent induction of a temporal retinal character is required for visual function
- Authors
- Hernández-Bejarano, M., Gestri, G., Monfries, C., Tucker, L., Dragomir, E.I., Bianco, I.H., Bovolenta, P., Wilson, S.W., Cavodeassi, F.
- Source
- Full text @ Development
|
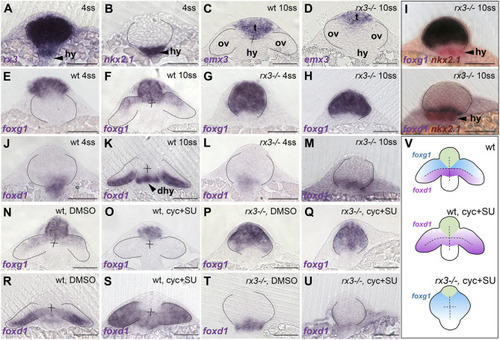
foxd1 expression is reduced in rx3?/? mutant optic vesicles and lost in rx3?/? upon combined abrogation of Shh and Fgf. (A-U) Frontal sections at the level of the forming telencephalon, optic vesicles and hypothalamus, with dorsal upwards; genotype, stage and/or treatment are indicated in the top right corner and the genes analysed are indicated in the bottom left corner. (A-D) Expression of rx3 (A) and nkx2.1 (B) in 4 ss wild-type/rx3?/? embryos (indistinguishable at this stage with a representative embryo shown) and emx3 in 10 ss wild-type (C) and rx3?/? (D) embryos, highlighting the prospective telencephalic (t), hypothalamic (hy) and eye-forming (ov) domains. (E-H,J-M) Expression of foxg1 (E-H) and foxd1 (J-M) in 4 ss (E,G,J,L) and 10 ss (F,H,K,M) embryos. (I) Double in situ hybridisation of foxd1 or foxg1 and nkx2.1 (red) to show the relationship between optic vesicles and the hypothalamus. (N-U) 10 ss stage embryos showing expression of foxg1 (N-Q) or foxd1 (R-U) in embryos treated with DMSO (N,P,R,T) or cyclopamine+SU5402 (O,Q,S,U). (V) Schematic representation of the conditions in R-U. Thirty to 50 embryos were treated and processed together per experiment and marker. rx3?/? embryos were recovered at the expected mendelian proportions and the phenotypes observed were fully penetrant. Scale bars: 100 µm. Dashed lines indicate the contour of the optic vesicles. dhy, dorsal hypothalamus; hy, hypothalamus; ov, optic vesicles; t, telencephalon. EXPRESSION / LABELING:
PHENOTYPE:
|
|
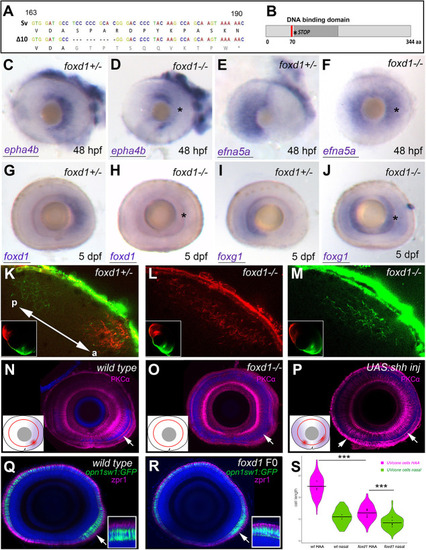
Loss of foxd1 results in retinae with expanded nasal and reduced temporal character. (A) foxd1 sequence comparison between nucleotides 163 and 190 of the open reading frame, highlighting the 10 bp deletion (?10, bottom row) in the foxd1cbm16 mutant. (B) Diagrammatic representation of the Foxd1 protein, depicting (in red) the deleted region in the cbm16 allele. The asterisks in A and B mark the position at which a newly generated stop codon is present. (C-J) Lateral views with nasal towards the left of 48 hpf (C-F) and 5 dpf (G-J) retinae, showing expression of nasal or temporal markers in foxd1?/? mutants and siblings. The markers assessed and the genotypes of the retinae are given in the bottom left and top right corners, respectively. Asterisks in D,F,H,J highlight altered expression in foxd1 mutants. Twenty-five to 30 embryos were processed together per marker. foxd1?/? embryos were recovered at the expected mendelian proportions and the phenotypes observed were fully penetrant. Genotype of imaged embryos was confirmed by molecular genotyping. (K-M) Dorsal views of the tectum of foxd1?/? mutant (L,M; n=12) and sibling (K; n=10) embryos, with nasal RGC arbours labelled using DiO (green) and temporal RGC arbours using DiI (red). Insets in K-M show dorsal views of the corresponding eye. Tectum and eye orientation in K-M are indicated by the double-headed arrow in K (a, anterior; p, posterior). (N-P) PKC? immunostaining (magenta) in lateral views with nasal towards the left of 8 dpf wild-type (N), foxd1?/? mutant (O) and tg{rx3:Gal4};UAS:Shh (P) eyes. Nuclei are counterstained using DAPI (blue). Insets in N-P are schematics of the eyes in the corresponding panels, highlighting the extent of temporal character (purple), as assessed by gene expression, and the areas enriched with PKC? (red). Arrows indicate the position of PKC? enrichment. (Q,R) Sagittal sections across wild-type (Q) and foxd1 crispant (R) eyes with nuclei stained using DAPI (blue), the outer photoreceptor segments stained using anti-Zpr1 antibodies (magenta) and UV cones expressing GFP [green, Tg(opn1sw1:GFP)] in 8 dpf larvae. (R) An example of a mild phenotype in comparison with other embryos with more severely reduced HAA labelling. (S) Violin plots showing differences in cone cell length between cells located in the HAA and nasal retina in wild type (n=3 eyes) and foxd1 crispants (n=6 eyes). Horizontal bars show the mean cell length for each condition; dots indicate the mean cell length for each individual eye (***P<0.001; one-way ANOVA) Scale bars: 100 µm. |
|
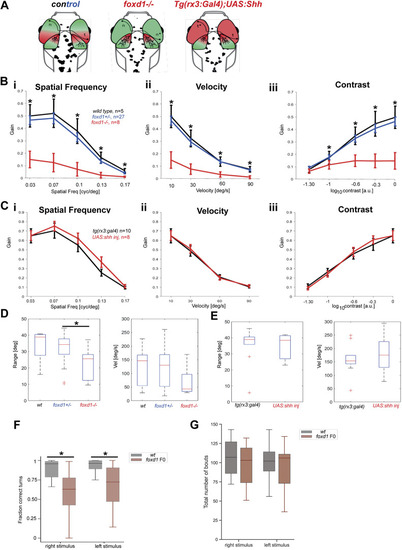
Optokinetic response assays reveal severe visual deficiencies in foxd1?/? but not tg{rx3:Gal4};UAS:Shh embryos. (A) Diagrammatic representation of the changes in naso-temporal pattern and retinotectal projections observed in the four categories of fish tested by optokinetic response (OKR). Genotype is provided at the top of each diagram. (B,C) Graphs detailing eye gain (expressed in a 0-1 range) in response to changes in spatial frequency (expressed in cycles per screen; Bi,Ci), grating velocity (expressed in degrees per second; Bii,Cii) and contrast (expressed in a 0-1 range; Biii,Ciii) in wild type (black, n=5 in B), foxd1+/? (blue, n=27 in B), foxd1?/? (red, n=8; in B), tg{rx3:Gal4} [black (rx3:gal4), n=10 in C] and tg{rx3:Gal4};UAS:Shh [red, (+UAS:shh), n=8 in C]. Graphical representation was generated using GraphPad Prism5 (data were analysed using two-way ANOVA; *P<0.001). (D,E) Range (expressed in degrees) of slow phase eye movement (left graphs) and peak velocity (expressed in degrees per second) during fast phase eye movement (right graphs) in foxd1?/? versus wild-type and foxd1+/? fish (D), and tg{rx3:Gal4} versus tg{rx3:Gal4};UAS:Shh fish (E) (*P<0.05; one-way ANOVA). (F) Fraction of correct turns for wild-type larvae (n=21) and foxd1 crispants (foxd1 F0, n=21) when subjected to left- and right-oriented whole-field motion stimuli. Only directional bouts (i.e. left- and rightward swims, without forward swims) were considered for quantification. (G) Total number of bouts for wild-type larvae (n=21) and foxd1 crispants (foxd1 F0, n=21) when subjected to left- and right-oriented whole-field motion stimuli. PHENOTYPE:
|
|
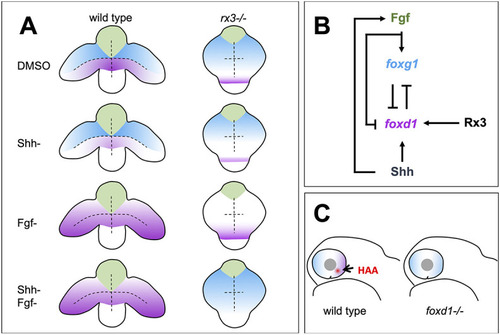
Summary of roles for Rx3, Fgfs and Shh in establishment of nasal and temporal character in the developing eye primordium. (A) Schematic representation of wild-type and rx3?/? embryos depicting the changes in foxg1 (blue) and foxd1 (magenta) expression upon abrogation of Shh and Fgf activities, singly or in combination. (B) Diagram detailing the proposed regulatory network controlling NT patterning. Shh promotes foxd1 expression in the ventral (future temporal) half of the optic primordium. Fgfs induce foxg1 in the dorsal (future nasal) optic primordium and repress foxd1 expression, contributing to its confinement ventrally. Cross-repression between Foxg1 and Foxd1 at the border between the dorsal and ventral halves of the eye primordium subsequently refines the naso-temporal subdivision. Although induction of shh and fgf8 expression occurs independently, fgf8 expression is lower in the absence of Shh signalling (Hernández-Bejarano et al., 2015), suggesting Hh signalling initially promotes Fgf signalling. Rx3 induces foxd1 expression and provides competence to optic vesicle cells to enhance foxd1 expression in prospective temporal retina in response to Shh signalling. (C) Schematic representation of a differentiated optic cup with nasal (blue) and temporal (magenta) character, and the HAA (red) highlighted. The nasal character is expanded and HAA and temporal character are absent in foxd1?/? mutants (right) when compared with the wild type (left). |