- Title
-
Zebrafish dazl regulates cystogenesis and germline stem cell specification during the primordial germ cell to germline stem cell transition
- Authors
- Bertho, S., Clapp, M., Banisch, T.U., Bandemer, J., Raz, E., Marlow, F.L.
- Source
- Full text @ Development
|
(A,A′) Single confocal plane of a 10-day larval gonad with DAPI-labeled nuclei (gray), GCs marked with Vasa (green), and F-actin with phalloidin (pink). Cystogenesis stages are boxed (white dotted lines). Solid white boxes delineate the focal plane showing the two- or four-cell stage cysts. (B-B″) Images showing a one-cell stage cyst with compact DNA (B) and a high cytoplasm/nucleus ratio (B′). Yellow dashed line indicates the nucleus. (C-C″) Division produces a two-cell stage cyst with two nuclei (yellow dashed line) surrounded by perinuclear Vasa. (D-D″) A four-cell cyst with four nuclei (yellow dashed line). (E-E″) Intercellular bridges (white arrow) in a 10-day larval gonad. (E′) XY view without Vasa (green). White arrow indicates the intercellular bridge. (E″) ZY view of the actin ring (white arrow). |
|
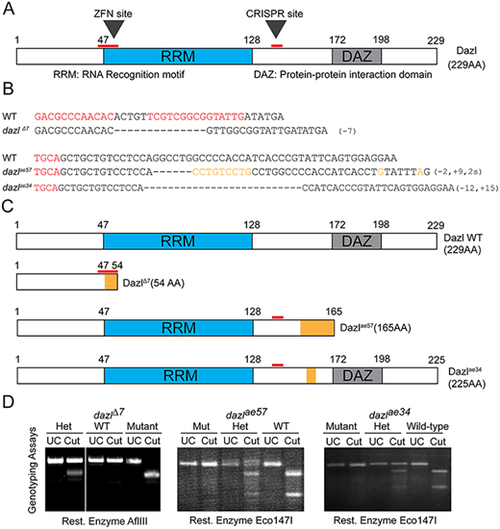
(A) Dazl protein diagram illustrating the RRM and DAZ domains with their respective amino acid regions. ZFN and CRISPR sites are indicated by red lines. (B) dazl alleles generated by ZFN (dazlΔ7) and CRISPR (dazlae57and dazlae34). Partial ZFNs and Cas9 binding sites are highlighted in red. Dashed lines represent deletions and orange denotes substitutions. (C) Diagram of alleles generated. The ZFN allele (top) causes a 7 bp deletion and leads to a premature stop codon eliminating all functional domains. dazlae57 (second from top) creates a 9 bp insertion and 2 bp substitution leading to a premature stop codon after the RRM domain. The third allele, induced a 12 bp deletion and 15 bp insertion causing an in-frame deletion between the RRM and DAZ domains. (D) Representative PCR/derived cleaved amplified polymorphic sequences genotyping assay for each allele. Heterozygotes harbor the wild-type allele (upper band) and the mutant allele (lower band) in the case of dazlΔ7. dazlae57 or dazlae34 heterozygotes harbor wild-type (lower band) and mutant alleles (upper band). |
|
(A-A″) Dazl is not detectable in PGCs marked with Vasa at 30 hpf. (A′,A″) Enlargement of the yellow dotted box in A with Dazl (A′, green) and Vasa (A″, magenta). (B-F″) Single confocal plane of representative 12-day dazlae57/+ and dazlae57/ae57 gonads (B-C″), and dazl+/+, dazlΔ7/+ or dazlΔ7/Δ7 14-day gonads (D-F″) immunostained with Dazl (green), Vasa (magenta) and DAPI (gray) as nuclear marker. Yellow dotted lines delineate GCs and blue lines outline somatic cells. (B-C″) Localization of Dazl in Vasa+ GC of dazlae57/+ (B-B″, n=1) and dazlae57/ae57 (C-C″, n=1). There was less abundant Dazl in dazlae57/ae57 gonads. (D-D″) Localization of Dazl in Vasa+ GC of dazl+/+ (n=1). Image is 63× without 2.5× zoom to show full gonad at resolution 512×512. (E-E″) Localization of Dazl in Vasa+ GC of dazlΔ7/+ (n=1). (F-F″) No detecatable Dazl in Vasa+ GC of dazlΔ7/Δ7 (n=2). Yellow dotted line delineates the gonad. (G-G″) Twenty-nine-day gonads labeled with Dazl (green) and Vasa (magenta). DAPI (gray) marks nuclei. Dazl protein was diffusely localized in the cytoplasm of the GCs. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Larval gonads labeled with Vasa (green), DAPI and F-actin (phalloidin). (A,A′,C,C′,E,E′,G,G′,I,I′) Single confocal plane of dazlae57/+ gonad showing cystogenesis between 8-14 days. (A,A′,C,C′,E,E′) Clustered Vasa+ individuals (white arrowheads) in the first step of cystogenesis with compaction and compartmentalization into irregular nuclear Vasa+ and Vasa− domains (C,C′,D,D′) at 8-10 days. Although initial compaction and compartmentalization occurs in mutants, wild-type GCs form cysts with multiple nuclei evident (E,E′, aqua arrowhead), whereas the pretransition morphology is observed in dazl mutants (F,F′). (G,G′) Amplification of GC numbers and round shaped nuclei with one to two nucleoli (yellow arrowheads) at 12 days (n=3). The shared tricellular junction is marked by a blue asterisk. (I,I′) Cyst cells with premeiotic nuclei and prominent nucleolus (white arrowhead). dazlae57/+ [8 days, n=3 (A,A′); 8 days, n=6 (C,C′); 10 days, n=11; 12 days, n=5; 14 days, n=6]. (B,B′,D,D′,F,F′,H,H′,J,J′) age-matched dazlae57/ae57 gonads. All dazlae57/ae57 germ cells end up as individuals with convoluted DNA (white arrows) lacking cyst organization. dazlae57/ae57 [8 days, n=6 (B,B′); 8 days, n=2 (D,D′); 10 days, n=5; 12 days, n=2; 14 days, n=7). (K) Quantification of categories between 8-14 days in dazlae57/+ and dazlae57/ae57 gonads. Categories match the corresponding box at the top right. (L) Quantification of cells/cyst at 8-14 days in dazlae57/+ and dazlae57/ae57 gonads. Cells in transition-amplification stage were not quantified because cell boundaries were not reliably distinguishable. Error bars represent s.d. PHENOTYPE:
|
|
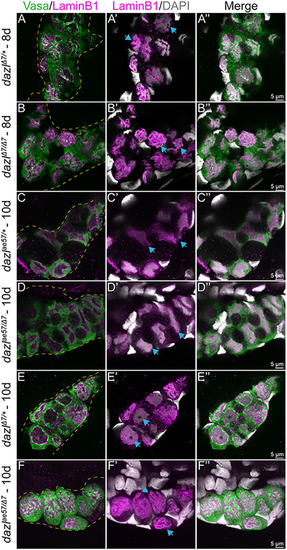
Single confocal plane of representative dazlΔ7/+ or dazlΔ7/Δ7 day 8 gonads and dazlae57/+, dazlΔ7/+ or dazlΔ7/ae57 at 10 days labeled with Vasa (green), LaminB1 (magenta; nuclear envelope,) and DAPI (gray; DNA). (A-A″) Vasa+ GC of dazlΔ7/+ (n=2) at day 8, with a folded raisin-like nuclear envelope. (B-B″) Vasa+ GC of dazlΔ7/Δ7 (n=3) at day 8, with an unfolded smooth nuclear envelope of Vasa+ GC of dazlae57/+ (C-C″, n=2) at 10 days. (D-D″) The change in nuclear appearance corresponds to amplification during cyst formation and is intact in dazlae57/Δ7. (E-E″) After the transition at 10 days, the nuclei of Vasa+ GC of dazlΔ7/+ remain smooth (n=2). The postmitotic GC nuclei also have a smooth nuclear membrane and a large nucleolus. (F-F″) Vasa+ GC of dazlae57/Δ7 (n=5) at 10 days. Mutant GCs are individuals with a folded nuclear membrane rather than a smooth morphology at 10 days. Yellow dotted line delineates the gonad. Blue arrows indicate each note for the corresponding panel. PHENOTYPE:
|
|
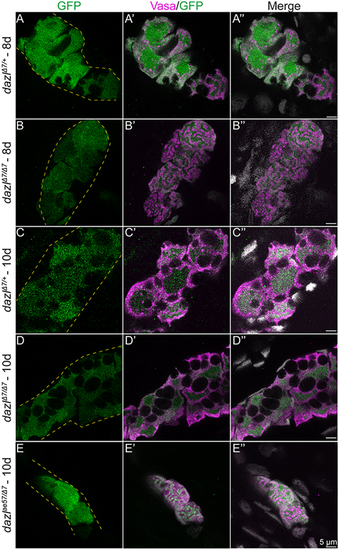
(A-E″) Single confocal plane of representative dazlΔ7/+ and dazlΔ7/Δ7 at day 8, and dazlΔ7/+, dazlΔ7/Δ7 and dazlae57/Δ7 gonads at day 10. Fish are transgenic for [ziwi:GFP], expressed zygotically in the germ cells. Gonads were immunostained with GFP (green, germ cells), Vasa (magenta) as a GC-specific cytoplasmic marker, and DAPI (gray; nuclei). (A-A″) GFP expression in Vasa+ GC of day 8 dazlΔ7/+ (n=2, B-B″) and dazlΔ7/Δ7 (n=2) gonads, and day 10 dazlΔ7/+ (n=2, C-C″), dazlΔ7/Δ7 (n=4, D-D″) and dazlae57/Δ7 (n=2, E-E″) gonads. Yellow dashed lines delineate the gonad. EXPRESSION / LABELING:
|
|
Single confocal plane of dazlae57/+ or dazlae57/ae57 8-14-day gonad labeled with Vasa and fluorescently conjugated phalloidin, which marks actin-rich structures and DAPI. (A,A′,C,C′, E,E′,G,G′) The actin-rich structures (white arrowheads) are present transiently in dazlae57/+ 8-12-day gonads. Number of gonads analyzed: 8 days, n=1 (A,A′); 10 days, n=1 (C,C′); 12 days, n=3 (E,E′); 14 days, n=2 (G,G′). (B,B′,D,D′,F,F′,H,H′) Actin-rich structures (white arrowheads) are present in 8-14-day dazl mutant GCs. (H,H′) At 14 days, actin-rich doublets are observed. Number of gonads analyzed: 8 days, n=1 (B,B′); 10 days, n=2 (D,D′); 12 days, n=2 (F,F′); 14 days, n=6 (H,H′). (I) Quantification of cells containing an actin-rich structure within each gonad. (J) Quantification of actin aggregates per cell. Error bars represent s.d. |
|
Single confocal plane of representative dazlae57/+ or dazlae57/ae57 10-14-day gonads labeled with fluorescently conjugated phalloidin (actin) depicting the actin ring. Each stage is represented with a sagittal view (XY) or maximum projection (ten planes of a z-stack). (A,A′,C,C′,E,E′) Actin rings are indicated by a blue arrow in dazlae57/+ 8-14-day gonads. Number of gonads analyzed: 10 days, n=3 (A,A′); 12 days, n=3 (C,C′); 14 days, n=3 (E,E′). (B,B′,D, D′,F,F′) Actin rings at 10 days in dazlae57/ae57 cells. At 12- and 14-days, actin rings are not maintained (blue arrows). Gonads analyzed: 10 days, n=4 (B,B′); 12 days, n=2 (D,D′); 14 days, n=3 (F,F′). (G) TEM image of day 14 wild-type gonad stitched from tiled images (magnification 700×). (H) Magnified image of (G, pink dashed line box) showing a ring canal connecting sister GCs within a cyst. (I) Higher magnification of H (pink dashed line box). (J) TEM image of 14-day dazl mutant gonad stitched from tiled images (magnification 700×). No cysts or ring canals were detected. Pink arrows indicate the centrioles and green arrows indicate the ring canal. Blue arrowheads indicate the germ cell membranes. PHENOTYPE:
|
|
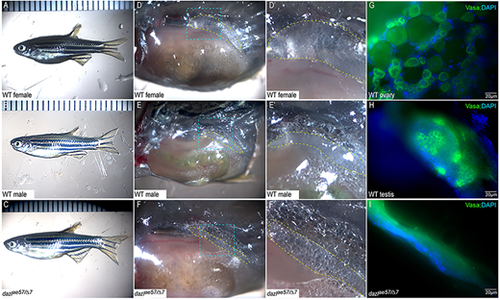
Zygotic dazl mutants are sterile males. (A-C) Overall morphology of representative adult wild-type female (A), male (B) and dazlΔ7/ae57 compound heterozygote (C). Males and females are distinguished based on secondary sex traits (color and tubercles on male fins). (D-F) Dissected trunks with the gonad region, indicated with yellow dashed lines, of wild-type female (D), male (E) and dazlΔ7/ae57 compound heterozygote (F). (D′-F′) Higher magnification views of blue outlined region in D-F. (D′,E′) Oocytes in females (D′) and sperm/testicular structures of males (E′). No identifiable gametes or GCs were detected in compound heterozygotes (F′). (G-I) Gonads labeled with Vasa reveal the oocytes (G), spermatocytes (H) and a gonad lacking GCs (I). PHENOTYPE:
|
|
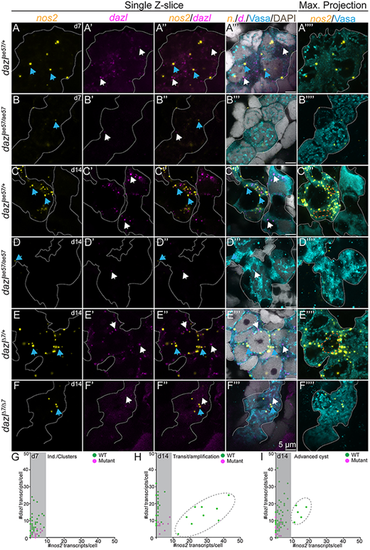
Absence of germline stem cells in dazl mutants. (A-F⁗) Single confocal plane of representative dazl+/+ or dazlae57/ae57 gonads at 7 days (individuals/clustered cells) and 14 days (transition/amplification), and dazl+/+ or dazlΔ7Δ7(advanced cyst) at 14 days labeled with nanos2 (yellow) or dazl (magenta) RNA probes, Vasa (cyan) and DAPI (gray). Sagittal view (XY) (left side) or maximum projection (right side) of 5-10 planes of a z-stack. (A-A⁗) Early nanos2 (blue arrows) and dazl (white arrows) foci in individuals/clustered cells in dazl+/+ at 7 days. (B-B⁗) Expression of nanos2 and dazl in individuals/clustered cells in dazlae57/ae57 at 7 days. (C-C⁗) Presence of abundant nanos2 foci during transition/amplification (orange dotted line surrounding the nuclear envelope). All GCs express dazl RNA. (D-D⁗) Scattered nanos2 and dazl foci in dazlae57/ae57 mutant cells that failed to progress beyond transition/amplification at day 14. (E-E⁗) Germline cysts in dazl+/+ with two populations: nanos2+ low-expressing cells (cells without an orange dashed line) and nanos2+ high-expressing cells (cells with an orange dotted line). (F-F⁗) nanos2 (blue arrows) foci in dazlΔ7Δ7 cells. (G) Quantification of nanos2 foci/cell compared with dazl foci/cell at 7 days (clustered stage). (H) Quantification of nanos2 foci/cell compared with dazl foci/cell at 14 days (transition/amplification stage). Dark gray dashed line encircles the nanos2+ high population. (I) Quantification of nanos2 foci/cell compared with dazl foci/cell at 14 days (advanced cyst stage). Dark gray dashed line encircles the nanos2+ high population. Gonads analyzed: 7 days, dazl+/+, n=5 (A-A⁗); 7 days, dazlae57/ae57, n=2 (B-B⁗); 14 days, dazl+/+ n=3 (C-C⁗); 14 days, dazlae57/ae57 n=2 (D-D⁗); 14 days, dazl+/+, n=4 (E-E⁗); 14 days, dazlΔ7Δ7, n=2 (F-F⁗). EXPRESSION / LABELING:
PHENOTYPE:
|
|
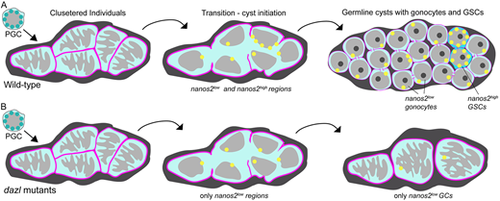
Schematics depicting the PGC to germline cysts with germline stem cell transition. (A) In wild-type gonads, individual PGCs cluster near one another and undergo dramatic nuclear rearrangements, possibly reflecting the onset of differentiation into germline stem or progenitor cells. During the transition step, the cell boundaries (pink) are not apparent as nanos2 (yellow) becomes detectable as discrete puncta with low and high regions of expression. Incomplete cytokinesis generates progenitors with round nuclei connected by intracellular bridges. Most cells express low levels of nanos2, whereas a few express high levels of nanos2, the likely GSCs. (B) In dazl mutants the first steps are intact. Mutant germ cells enter the transition state and express low levels of nanos2, but not high levels. As gonad development ensues, only individual GCs are detected in mutants and no GSCs are specified. It is unclear whether GCs revert to the pre-transition state or whether they are lost; however, blocking cell death does not prevent the germline loss or sterility of dazl mutants. Thus, Dazl is essential for germline cyst formation and GSC specification, either directly or indirectly. |