- Title
-
Spen deficiency interferes with Connexin 43 expression and leads to heart failure in zebrafish
- Authors
- Rattka, M., Westphal, S., Gahr, B.M., Just, S., Rottbauer, W.
- Source
- Full text @ J. Mol. Cell. Cardiol.
|
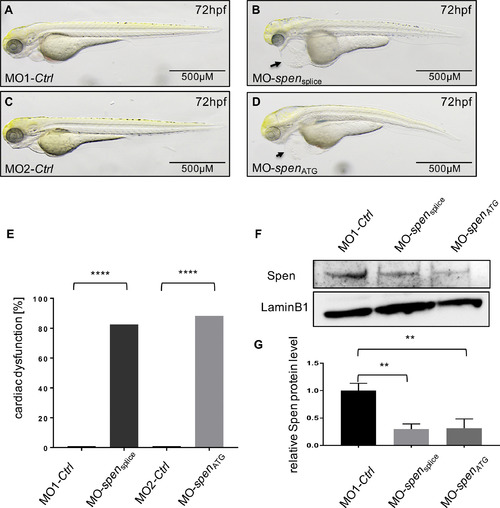
Lateral view of Spen-deficient (B,D) and control-injected embryos (A,C) at 72 h post fertilization (hpf). Spen deficiency results in development of a large pericardial edema at 72 hpf (B,D black arrow), while the controls don't display cardiac abnormalities (A,C). (E) 86% (190/221) MO-spensplice- and 79% (145/184) MO-spenATG- injected embryos display the same cardiac phenotype. Embryos with pericardial edema, bradycardia, and impaired contractility were counted as affected. (F) Efficacy of morpholino-mediated knockdown experiments was confirmed by Western Blot analysis; representative immunoblots are shown. (G) Relative quantification of Spen protein levels (n = 3). Images (A,B,C,D,F) have been cropped. Assessment of significance by chi-squared test (E) or t-test (G). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. PHENOTYPE:
|
|
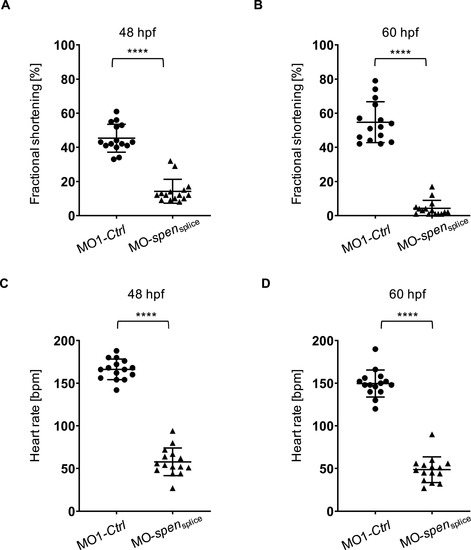
(A,B) Fractional shortening of spen morphants and controls at 48 hpf and 60 hpf. Impairment of ventricular fractional shortening in Spen-deficient embryos starts at 48 hpf (12 [10%?15%] vs. 43 [41%?53%], n = 15) and becomes more severe at 60 hpf (3 [1%?5%] vs. 52 [44%?65%], n = 15). (C,D) Heart rate (in beats per minute) is severely reduced in spen morphants compared to control-injected fish at 48 hpf (49 ± 15 vs. 150 ± 15, n = 15) and 60 hpf (58 ± 16 vs. 166 ± 12, n = 15). A?D: Assessment of significance by Wilcoxon-Mann-Whitney test. ****p < 0.0001. |
|
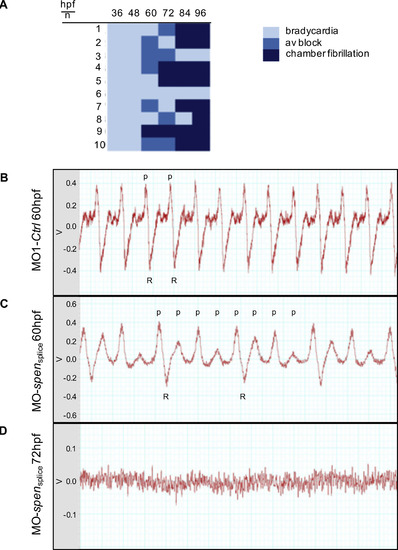
(A) Overview of the heart rhythm phenotype of ten Spen-deficient embryos at different developmental time points. (hpf: hours post fertilization). (B-D) Zebrafish electrocardiograms. While in the controls every atrial excitation (p: p-wave) is followed by a ventricular potential (R: R-wave); (B), spen morphants show atrioventricular conduction abnormalities, as not every p-wave is followed by an R-wave (C). At 72 hpf only an uncoordinated atrial and ventricular electric activity can be documented, resembling atrial and ventricular fibrillation (D). V: Volt; Images (B,C,D) have been cropped. PHENOTYPE:
|
|
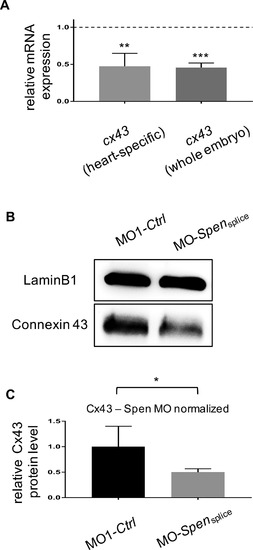
(A) By screening for dysregulated genes via a cardiovascular disease assay we identified gap junction protein Connexin 43 (Cx43) to be downregulated (supplementary material Fig. A.3A) in Spen-deficient embryos 72 hpf, which was confirmed by cx43-specific qPCR in Spen-deficient hearts (0.47 ± 0.10, p = 0.0063, n = 3) as well as Spen-deficient embryos (0.46 ± 0.04, p = 0.0001, n = 3). (B) Western Blot analysis verifies downregulation of Cx43 in Spen-deficient embryos on the protein level. (C) Quantification of Cx43 levels in Western Blots of Spen-deficient and control embryos (n = 3). Images (B) have been cropped. A, C: Assessment of significance by t-test. *p < 0.05, **p < 0.01, ***p < 0.001. EXPRESSION / LABELING:
PHENOTYPE:
|
|
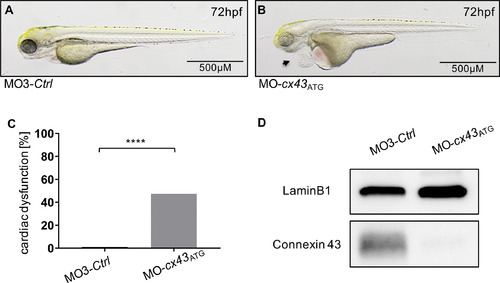
(A,B) Lateral view of Cx43-deficient (B) and control-injected embryos (A) at 72 hpf. Cx43 deficiency results in development of a large pericardial edema at 72 hpf (B, black arrow) while the controls don't display cardiac abnormalities (A). (C) 47% (47/99) MO-cx43ATG- injected embryos displayed a heart failure and arrhythmia phenotype. Embryos with pericardial edema, bradycardia, and impaired contractility were counted as affected. (D) Efficacy of morpholino-mediated knockdown experiments was confirmed by Western Blot analysis. Images (A, B) have been cropped. C: Assessment of significance by chi-squared test. ****p < 0.0001. PHENOTYPE:
|
|
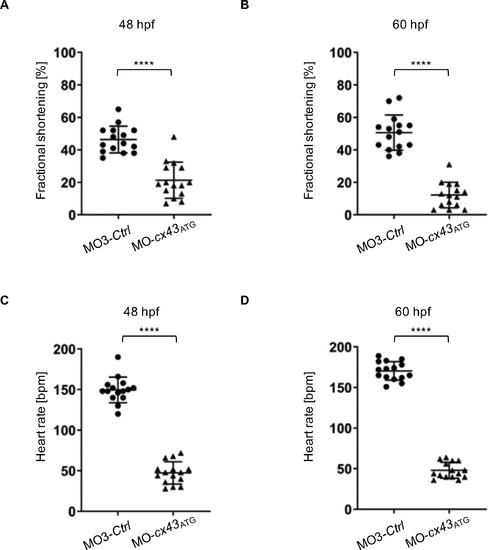
(A,B) Fractional shortening of connexin43 (cx43) morphants and controls at 48 hpf and 60 hpf. Impaired ventricular fractional shortening in Cx43-deficient embryos starts at 48 hpf (19 [13%?29%] vs. 44 [39%?52%], n = 15) and becomes more severe at 60 hpf (12 [6%?19%] vs. 51 [42%?55%], n = 15). (C,D) Heart rate (in beats per minute) is severely reduced in cx43 morphants compared to control-injected fish at 48 hpf (47 ± 14 vs. 150 ± 16, n = 15) and 60 hpf (48 ± 15 vs. 170 ± 11, n = 15). A-D: Assessment of significance by Wilcoxon-Mann-Whitney test. ****p < 0.0001. |
|
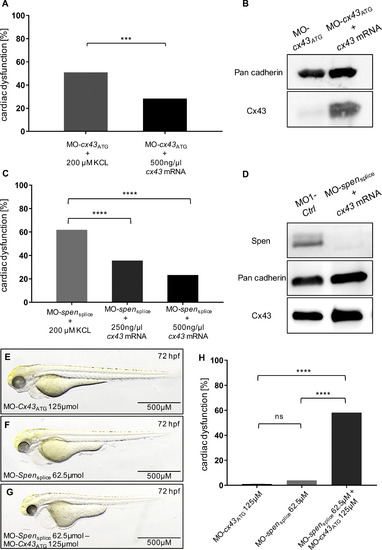
(A) Injection of 500 ng/?g cx43 mRNA in MO-cx43ATG-injected zebrafish leads to 28% (21/78, three independent experiment) embryos displaying heart failure and arrhythmia, while MO-cx43ATG-injection results in 51% (55/108, three independent experiments; p = 0.0024) affected embryos. (C) MO-spensplice injection resulted in 62% (78/126, three independent experiments) affected embryos, while co-injection with either 250 ng/?l or 500 ng/?l cx43 mRNA significantly decreased the amount of diseased embryos to 36% (52/146, three independent experiments, p < 0.0001) and 23% (28/120, three independent experiments, p < 0.0001), respectively. Embryos with pericardial edema, bradycardia, and impaired contractility were counted as affected. (B, D) Western Blot analysis of MO-cx43ATG, MO-cx43ATG + cx43 mRNA and MO1-Ctrl and MO-spensplice + cx43 mRNA injected fish confirms reconstitution of Cx43 levels in cx43- and spen morphants, respectively. (E-G) Lateral views of embryos injected with sub-phenotypic doses of MO-cx43ATG (E), MO-spensplice (F) and a co-injected embryo (G) at 72 h post fertilization (hfp). (H) Sensitizing experiment. While injection of sub-phenotypic doses of morpholino did not cause relevant phenotypic abnormalities 72 hpf, co-injection resulted in a significant supra-additive effect (79/136, three independent experiments; p < 0.0001, respectively), suggesting molecular crosstalk between Spen and Cx43. Images (B,D,E,F,G) have been cropped. A, C, H: Assessment of significance by chi-squared test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. PHENOTYPE:
|
Reprinted from Journal of Molecular and Cellular Cardiology, 155, Rattka, M., Westphal, S., Gahr, B.M., Just, S., Rottbauer, W., Spen deficiency interferes with Connexin 43 expression and leads to heart failure in zebrafish, 25-35, Copyright (2021) with permission from Elsevier. Full text @ J. Mol. Cell. Cardiol.