- Title
-
Zebrafish heart regenerates after chemoptogenetic cardiomyocyte depletion
- Authors
- Missinato, M.A., Zuppo, D.A., Watkins, S.C., Bruchez, M.P., Tsang, M.
- Source
- Full text @ Dev. Dyn.
|
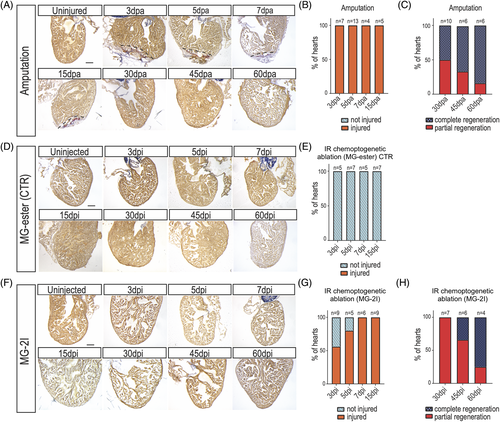
Zebrafish regenerate after cardiomyocyte chemoptogenetic ablation. A, AFOG staining in WT adult zebrafish hearts before and after ventricular apex amputation. (dpa) = days post amputation. Black dashed lines delineate injured area. B, Graph representing the percentage of hearts that were injured (orange) at multiple time points. Percentages were calculated upon sectioning and AFOG staining. 100% of the hearts undergoing amputation were injured, showing the efficiency of the surgery. C, Graph reporting the percentage of hearts that were completely (blue) or partially (red) regenerated at 30-, 45-, and 60 dpa. At 30 dpa, 50% of the fish were completely regenerated, while at 60 dpa almost all the fish showed complete regeneration. D, AFOG staining of Tg(myl7:fapdl5-cerulean) hearts after injection of control MG-ester and IR light treatment at several time points. (dpi) = days post injection. Hearts appeared normal at every time point studied. Damage was determined as fraction of myocyte area of the total ventricular area. E, Graph showing that upon injection of control MG-ester and IR light treatment. F, AFOG staining of Tg(myl7:fapdl5-cerulean) hearts after injection of MG-2I and IR light treatment at several time points. Visible loss of cardiomyocytes was detected as early as 3?dpi. G, Graph representing the percentage of hearts that were injured (orange) or not injured (light blue) at several time points after MG-2I injection. At 3 dpi only 50% of the hearts showed injury, but by 7 dpi, all the hearts showed injury. H, Graph representing the percentage of hearts that show complete or partial regeneration at later time points. Total number of hearts analyzed are indicated. Scale bars = 100??m |
|
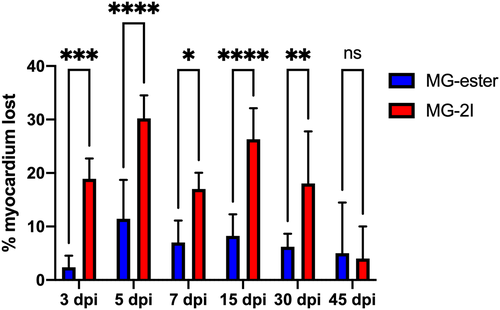
Chemoptogenetic damage causes loss of myocardium after MG-2I treatment. Regions of Interest were taken from WT uninjured, Tg(myl7:fapdl1-cerulean) MG-ester-, and Tg(myl7:fapdl1-cerulean) MG-2I-treated hearts after AFOG staining and intact myocardium was measured using Threshold Particle Analysis (Image J). Myocardial area from MG-ester and MG-2I hearts was normalized to WT uninjured hearts to generate the amount of tissue lost as a percentage. MG-2I-treated hearts showed significant loss of myocardium at 3 dpi and 5 dpi (compared to MG-ester controls), and also regenerated this damage by 45-60dpi. A minimum of three hearts were used per condition with the following as the total n values: WT uninjured (n = 6), MG-ester (3 dpi (n = 5), ?5 dpi (n = 5), ?7 dpi (n = 5), ?15 dpi (n = 5), ?30 dpi (n = 5), ?45 dpi (n = 3)), and MG-2I (3 dpi (n = 5), ?5 dpi (n = 5), ?7 dpi (n = 6), ?15 dpi (n = 6), ?30 dpi (n = 5), ?45 dpi (n = 3)). *P? |
|
Myocardium integrity was reduced after MG-2I treatment. Fluorescent in situ hybridization was used to assess the integrity of the myocardium after different types of injury. A representative image of uninjured heart (A?D; n = 5), 3 dpa (E?H; n = 3), control PBS treated Tg(myl7:fapdl1-cerulean) at 3 dpi (I?L and U, U?; n = 4), MG-2I treated Tg(myl7:fapdl1-cerulean) at 3 dpi (M?P and V, V?; n = 3) and 5 dpi (Q?T and W, W?; n = 4) are shown. Uniform distribution of myl7 were noted in uninjured and PBS control treated hearts at 3 dpi (A?D and E?H). Absence of myl7 were detected in 3 dpi (M?P, V) and 5 dpi (Q?T, W) hearts treated with MG-2I and infrared light that indicated loss of trabeculated myocardium and damage to the compact myocardium as marked by asterisks. Scale bar = 50??m |
|
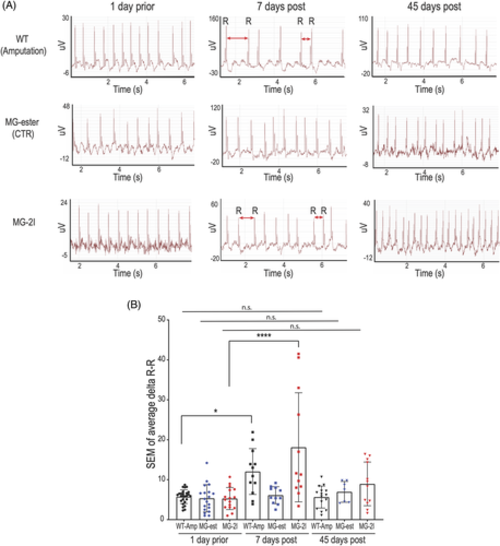
Chemoptogenetic cardiomyocyte ablation causes arrhythmia. A, Representative electrocardiograms (ECG) of adult zebrafish before and after amputation or ablation. One?day prior amputation or ablation (left panels), hearts showed normal heart beat rhythm without arrhythmic events. Seven?days post amputation or MG-2I injection and IR light treatment, several zebrafish showed arrhythmic events (central panels) Red double heads arrows indicate longer or shorter R-R intervals. Importantly, injection and irradiation of control MG-ester did not cause arrhythmia (central panels). Forty-five?days post injury (right panels), ECG were comparable to uninjured hearts, suggesting that all the hearts have recovered. B, Graph quantifying the ECG. At least n = 7 for each group. *P? |
|
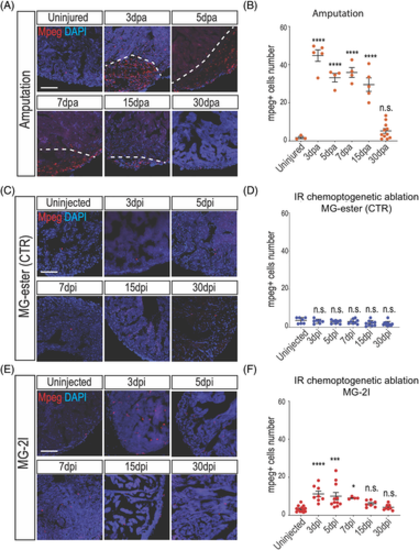
Macrophages are activated after cardiomyocyte chemoptogenetic ablation. A, Mpeg and DAPI staining before and at multiple time points after ventricular apex resection. White dashed lines indicate injury area. B, Quantification of mpeg+ cells inside the injury after amputation. Between 3- and 15 dpa, macrophages were detected inside the injured area. By 30 dpa, the number of macrophages were similar to the uninjured hearts. At least n = 3 for each group. ****P? |
|
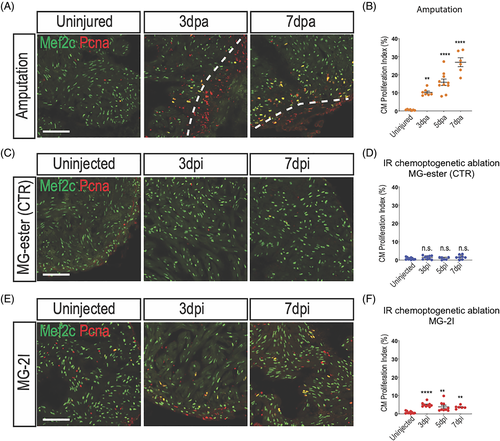
Cardiomyocyte proliferate after chemoptogenetic ablation. A, Mef2c and Pcna staining in amputated ventricles. White dashed lines indicate injury area. Amputation induced cardiomyocyte proliferation. B, Graph showing the percentage of proliferating cardiomyocytes before and after amputation. Cardiomyocyte proliferation started at 3 dpa and peaked at 7 dpa. **P? |
|
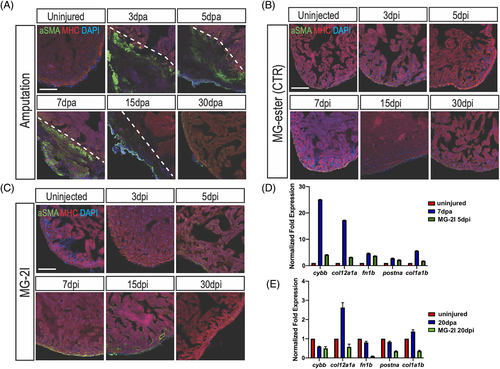
Myofibroblasts are mildly activated upon cardiomyocyte chemoptogenetic ablation. A, Activated fibroblasts (?SMA) and MHC (cardiac muscle) immunostaining before and after ventricular amputation at several time points. In uninjured ventricles ?SMA staining is not detectable. After amputation, ?SMA staining is visible inside the injury but by 30 dpa activated fibroblasts were not detected. B, Tg(myl7:fapdl5-cerulean) hearts injected with control MG-ester and treated with near IR-light, stained for ?SMA and MHC. ?SMA was not detected at any time point. C, In chemoptogenetic ablated hearts, myofibroblasts are weakly activated starting at 5 dpi, until 15 dpi. At least n = 3 for each time point. Q-PCR of cytochrome b, (cybb), collagen (col12a1a and col1a1b), fibronectin (fn1b), and periostin a (postna) in amputated and ablated hearts at 7?days and 5?days (D) and at 20?days (E). Scale bar = 100??m |
|
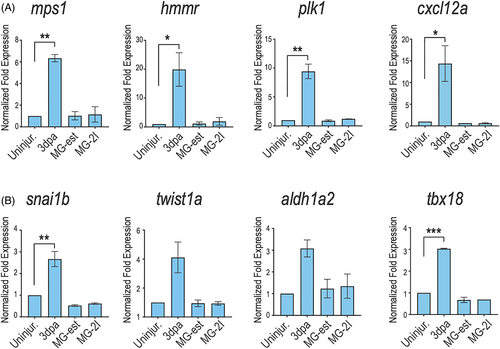
Several cardiac regeneration markers are not reactivated upon cardiomyocyte chemoptogenetic ablation. A, Q-PCR for several cardiac regeneration markers (mps1, hmmr, plk1, cxcl12a) at 3?days after amputation or genetic ablation. Increased expression of regeneration genes was noted in amputation model but not upon ROS-induced cardiomyocyte ablation. B, Q-PCR for epicardial markers (snai1b, twist1a, aldh1a2, and tbx18) at 3?days after injury. Amputation induced epicardial gene expression at 3?days, but ROS-induced ablation did not. *P? |