- Title
-
Pharmacological restoration of visual function in a zebrafish model of von-Hippel Lindau disease
- Authors
- Ward, R., Ali, Z., Slater, K., Reynolds, A.L., Jensen, L.D., Kennedy, B.N.
- Source
- Full text @ Dev. Biol.
|
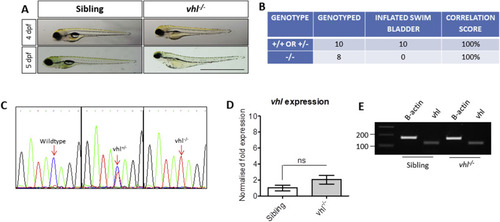
Characterisation of vhl?/? zebrafish. A) Gross morphology of 4 and 5 dpf vhl larvae highlighting the absence of a swim bladder, plus yolk and pericardial oedema in vhl-/- larvae. Scale bar?=?2?mm. B) Phenotype-Genotype correlations in offspring generated from vhl ± incrosses. DNA sequencing of PCR amplicons from genomic DNA confirmed a positive correlation between swim bladder uninflation and vhl?/? genotype. This strategy was used to select vhl?/? larvae for all further experimentation. C) Sequencing chromatograms illustrating the single nucleotide polymorphism (C?>?T) in the vhl sequence resulting in a premature STOP codon. D) Real time PCR analysis of vhl expression in vhl?/? larval eyes in comparison to siblings (+/? or +/+). ns = not significant when p < 0.05. E) Reverse transcriptase PCR highlighting vhl expression in vhl?/? and sibling eyes at 5 dpf. |
|
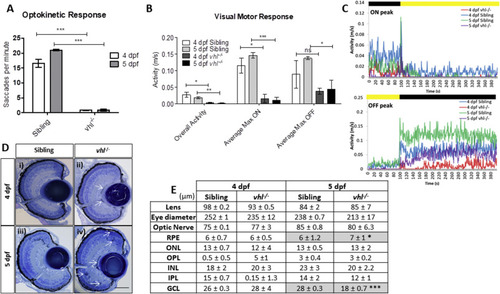
vhl?/? larvae display retinal abnormalities and impaired visual function. vhl?/?larvae possess A) reduced visual capacity in response to a moving striped stimulus (OKR) n?=?3, 12 technical replicates per treatment group per experiment. B) reduced movement in response to changes in light intensity (VMR) and C) VMR chromatographs illustrating average max ON/OFF peaks. n?=?3, 12 technical replicates per treatment group per experiment. D) vhl?/? larvae display defects in retinal arrangement at 5 dpf. Representative light microscopy images of retinal sections from i) 4 dpf sibling control, ii) 4 dpf vhl?/? iii) 5 dpf sibling control and iv) extensive retinal damage, thought to be fluid accumulation present in 5 dpf vhl?/? larvae (white arrows). Scale bar?=?50??m. E) Morphometric analysis based on 3 independent measurements from 3 larvae per group. Values represent mean?±?standard deviation of retinal layer thickness in ?m. No statistically significant differences is observed between retinal layers of sibling and vhl?/? at 4 dpf. The retinal layers analysed include Retinal Pigment Epithelium (RPE), Outer Nuclear Layer (ONL), Outer Plexiform Layer (OPL), Inner Nuclear Layer (INL), Inner plexiform Layer (IPL) and Ganglion Cell Layer. |
|
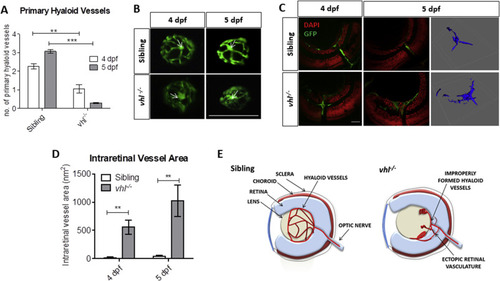
vhl?/? zebrafish larvae possess ocular vascular abnormalities at 4 and 5 dpf. A) Primary hyaloid vessel number at 4 and 5 dpf. vhl?/? larvae highlight a significant reduction in hyaloid branches at 4 and 5 dpf compared to sibling control. Data is representative of 3 biological replicates. N?=?10 larvae per group per biological replicate. B) Fluorescent images of fli1:EGFP hyaloid vessels on sibling and vhl?/- dissected lenses at 4 and 5 dpf. White arrows illustrate central point from which primary branches are quantified. Scale bar?=?200??m. C) Confocal imaging revealed abnormal retinal neovascularisation (white arrows) at 4 and 5 dpf through the inner plexiform layer (IPL) and ganglion cell layer (GCL) compared to sibling control. Z-stacks confirmed aberrant vessel growth (blue) through the retina. Scale bar?=?20??m. D) Intraretinal vessel area is significantly increased in vhl?/? at both 4 and 5 dpf. N?=?5 larvae per group E) Schematic of proposed ocular blood vessel formation in vhl?/? zebrafish larvae at 5 dpf. |
|
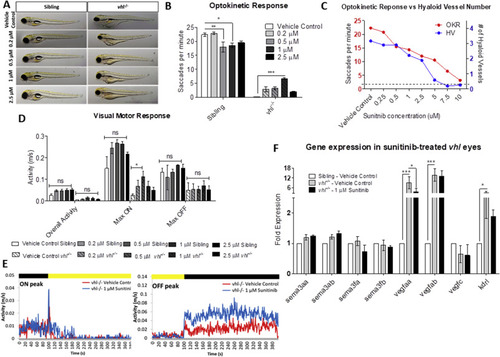
Sunitinib malate significantly improves visual function and reduces VEGF expression in vhl?/? larvae. A) Gross morphology of sibling and vhl-/- larvae treated with increasing doses of sunitinib malate at 5 dpf. Treatment reduces yolk and cardiac oedema but does not result in swim bladder inflation. B) All concentrations of sunitinib tested increase OKR visual function at 5 dpf with 1??M sunitinib malate highlighting a statistically significant improvement. n?=?3, 12 technical replicates per experiment. C) Correlation between hyaloid vessel number and visual function in 5 dpf wildtype larvae treated with sunitinib malate. n?=?3 with 10 technical replicates per experiment. Dotted line denotes vhl?/? visual function and number of primary hyaloid branches at 5 dpf. D) VMR Max ?ON? response is significantly increased dose dependently in larvae treated with 0.2??M and 0.5??M Sunitinib. n?=?3, 12 technical replicates per treatment group per experiment. E) VMR chromatograms illustrating the average Max ON and Max OFF peaks after treatment. F) Expression of pro- and anti-angiogenic vhl?/? larval eyes following sunitinib treatment. |
|
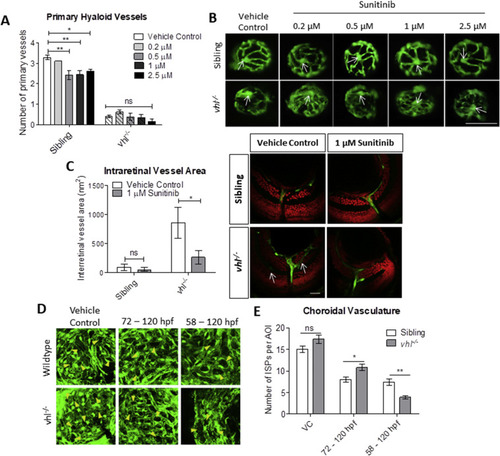
Sunitinib malate improves hyaloid vessel patterning, reduces retinal neovascularisation and inhibits excessive development of the choriocapillaris in vhl?/? larvae. A) Treatment from 58 hpf does not significantly increase vhl?/? primary hyaloid vessel number at 5 dpf. B) Representative images highlighting an overall improvement of vessel patterning following treatment with sunitinib from 58 hpf. White arrows denote central point from which primary blood vessels are counted. n?=?3, 12 technical replicates per group per experiment. C) Reduction of ectopic intraretinal vasculature following treatment with 1??M sunitinib. White arrows highlighting ectopic intraretinal vessels. Quantification of n?=?5 larvae per treatment group. Scale bar?=?20??m. D) Z-stack projections of the sub-retinal choriocapillaris vasculature (endothelial cells shown in green) of Tg(fli1:EGFP) or vhl?/?(fli1:EGFP) at 120 hpf following treatment for 72 or 48?h as indicated. Yellow arrowheads point to interstitial pillars (ISPs). E) Quantification of the number of interstitial pillars per area of interest (AOI). n?=?10 larvae. PHENOTYPE:
|
Reprinted from Developmental Biology, 457(2), Ward, R., Ali, Z., Slater, K., Reynolds, A.L., Jensen, L.D., Kennedy, B.N., Pharmacological restoration of visual function in a zebrafish model of von-Hippel Lindau disease, 226-234, Copyright (2019) with permission from Elsevier. Full text @ Dev. Biol.