- Title
-
The effector of Hippo signaling, Taz, is required for formation of the micropyle and fertilization in zebrafish
- Authors
- Yi, X., Yu, J., Ma, C., Dong, G., Shi, W., Li, H., Li, L., Luo, L., Sampath, K., Ruan, H., Huang, H.
- Source
- Full text @ PLoS Genet.
|
Some homozygous taz mutants can survive to adulthood. (A-C) Schematic of CRISPR/Cas9 knock-out of taz. The target site (shown in blue) is located in exon 1 of taz, and the PAM region is shown in red (A). Two mutant alleles with small deletions, taz Δ10 and taz Δ1, were obtained (B), both of which result in frame-shifts of the taz open reading frame and truncated proteins (C). aa, amino acid; TEAD BD, Tead transcription factor binding domain (light blue); WW, dual tryptophan motif (green); TAD, transactivation domain (purple); PDZ domain (blue). (D) Western blot analysis shows Taz protein in wild type but not in tazΔ10/ Δ10 mutant embryos. β-Tubulin is used as a loading control. (E, F) Similar to wild type embryos (E), tazΔ10/Δ10 embryos are largely normal at 4.5 dpf, except for a smaller inflated swim bladder and mild pericardial edema (F). (G) Survival curve of tazΔ10/Δ10 in heterozygote intercrosses from larval to adult stages (shown as % survivors, from two independent experiments). The red dashed line indicates the expected 25% homozygous mutants in accordance with Mendelian segregation. Number of larvae/adults at each stage: 7 dpf (59/228), 15 dpf (37/200), 30 dpf (20/182), 60 dpf (22/216) and 120 dpf (10/174). Black arrow, swim bladder; black arrowhead, pericardium. Scale bar, 500 μm. |
|
Loss of maternal Taz results in failure of eggs to develop. (A-D) 3 hpf embryos from crosses of a wild type female and male (A), wild type female with tazΔ10/Δ10 male (B), tazΔ10/Δ10 female with wild type male (C) and tazΔ1/Δ1 female with wild type male (D). Embryos from tazΔ10/Δ10 or tazΔ1/Δ1 females are arrested at the 1-cell stage. (E) Embryos from crosses of wild type female and male, wild type female with tazΔ10/Δ10 male, and tazΔ10/Δ10 female with wild type male are shown at 1-cell, 2-cell, 4-cell and 8-cell stages. Most embryos produced by tazΔ10/Δ10 females remain at the 1-cell stage, and a few initiate irregular cleavage planes at wild type 4-cell and 8-cell stage. (F-I) In situ hybridization to detect taz mRNA shows that taz transcripts are found in oocytes and follicle cells in ovary sections (F), and abundant expression in embryos at the 1-cell stage (H) when compared to sense probe control (I). Nonspecific signal is detectable in oocytes at stage I and II while using sense probe as control (G). Oocytes at stages I, II, III are shown; green arrow, the oocyte cortex; green arrowhead, follicle cells. Scale bar, 1 mm in A-D, 0.5 mm in E and 100 μm in F-I. |
|
Oogenesis and oocyte polarity seems normal in taz-/- zebrafish females. (A-D) Lateral views of dissected adult wild type and taz-/- ovaries. Similar to wild type (A), the taz mutant ovary (B) is grossly normal, and taz mutant oocytes (D) are similar to wild type (C) in size and numbers. (E-F) Haematoxylin and eosin (HE) stained sections show no apparent morphological difference in oogenesis between wild type (E) and taz-/-ovaries (F). Oocytes at stages I-IV are marked; n, nucleus; green box, CG (cortical granules). (G-J) In situ hybridization on sectioned ovaries to detect oocyte polarity markers show that animal-vegetal polarity is established in taz-/- oocytes similar to wild type oocytes. The Balbiani body, and subsequently, vegetal pole labeled by expression of dazl in wild type and taz-/- are normal in primary (G, H) and stage III (I, J) oocytes, and the animal pole, marked by cyclinB transcripts, is properly established in both wild type and taz-/-oocytes (I, J). Dark blue arrowhead, Balbiani body; dark blue arrow, vegetal pole; pink arrow, animal pole. Scale bar, 1 mm in A-D, 100 μm in E-F, and 50 μm in G-J. |
|
taz is essential for formation of the micropyle and fertilization. (A-D) At 15 minutes post fertilization, Coomassie Blue staining shows a single hole, the micropyle, in the chorion above the animal pole of wild type eggs (A, n = 92), but not in taz-/- eggs (B, n = 131). Upon activation, a cytoplasmic extension towards the micropyle is observed in wild type eggs (C, n = 162). By contrast, no cytoplasmic projection is observed in taz-/- eggs (D, n = 142). Insets in A and C are magnified images of the area within the pink circle. (E-F’) Two DAPI-stained pronuclei and one Actin-rich polar body are observed in a fertilized wild type eggs at 10 minutes post fertilization (E, E’, n = 61/63), whereas activated taz-/- eggs show one pronucleus and one polar body (F, F’, n = 47/47), suggesting that taz-/- egg is not fertilized, and the second polar body is extruded. White boxes in E to F denote regions magnified in E’ to F’. Red arrow, polar body; light blue arrow, pronucleus; AP, animal pole; VP, vegetal pole. Scale bar, 0.5 mm in A-B, 0.25 mm in C-D, 50 μm in insets in A and C, 100 μm in E-F and 20 μm in E’-F’. PHENOTYPE:
|
|
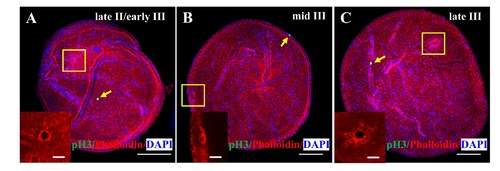
Taz is highly enriched in micropylar cells. (A-C’”) Immunofluorescence of Taz and β-Catenin in sectioned wild type oocytes at various stages: late II/ early III (A-A’”, n = 14), mid III (B-B’”, n = 14) and late III (C-C’”, n = 13). β-Catenin and DAPI label the cell membrane and nucleus, respectively. While basal levels are detected in follicle cells, in the micropylar cell high levels of Taz are detected, predominantly in the nucleus (A-C, A”-C”). Taz expression levels decrease as the micropylar cell develops (A-C, A”-C”). The micropylar cell undergoes dramatic shape change from flattened to ‘nail’-like to perforate the developing vitelline envelope (A’-C’, A”-C”). Bright field images show the gradual invagination of the vitelline envelope by the protruding micropylar cell (A’”-C’”). (D-E”) Immunofluorescence of Taz and β-Catenin in sectioned wild type and taz-/- oocytes at stage III after in situ hybridization to detect cyclinB. In wild type ovaries, the high Taz expressing micropylar cell sits on the top of the oocyte animal pole, marked by cyclinB, and perforates the vitelline envelope (D-D”, n = 9). No Taz is detectable in taz-/- oocyte (E), and neither the micropylar cell around the animal pole nor an invagination on the vitelline envelope is observed in mutant oocytes (E-E”, n = 11). (F-G) Immunofluorescence to detect Taz and in situ hybridization for cyclinB in wild type and taz-/- oocytes at stage III. A single high Taz expressing micropylar cell is located at the animal pole of wild type oocyte (F, n = 13), but is not found in taz-/- ovaries (G, n = 17). Two stage I oocytes, ubiquitously expressing cyclinB, are found adjacent to the stage III oocyte in figure F. White arrow, the micropylar cell indicated by high Taz expression. Scale bar, 100 μm in F and G and 10 μm in others. |
|
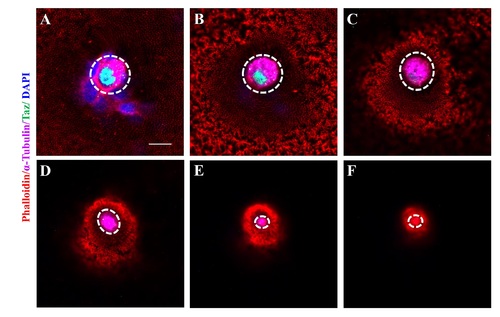
The nucleus in the micropylar cell is composed of two closely juxtaposed nuclei. (A-C’”) Single confocal plane of immunofluorescence of Taz and β-Catenin in whole mount wild type oocytes at three stages: late II/ early III (A-A’”), mid III (B-B’”) and late III (C-C’”) shows β-Catenin and DAPI in the cell membrane and nucleus respectively. The nucleus in most micropylar cells (57/60) is composed of two closely juxtaposed nuclei. (D-F’”) Similarly, single confocal planes of immunofluorescence of Taz and Nup107 (nuclear membrane marker) in stage late II/ early III (D-D’”), mid III (E-E’”) and late III (F-F’”) oocytes show two closely juxtaposed nuclei surrounded by continuous nuclear membranes in the micropylar cell (n = 32 oocytes). Scale bar, 10 μm. |
|
Taz may regulate cytoskeletal dynamics in the micropylar cell. (A-F’”) Immunofluorescence shows Taz and F-actin (A-C’”, n = 32) or α-Tubulin (D-F’”, n = 19) in sectioned ovaries; besides the invagination on the vitelline envelope, the expression of Taz and the shape of the micropylar cell changes with the growth of the micropylar cell; Phalloidin labelled F-actin bundles are found gradually deposited in the leading tip in the cytoplasmic extension of the micropylar cell and the part of oocyte cortex contacting the micropylar cell (A-C’”), and α-Tubulin is enriched in the cytoplasmic extension of the micropylar cell (D-F’”). Scale bar, 10 μm. |
|
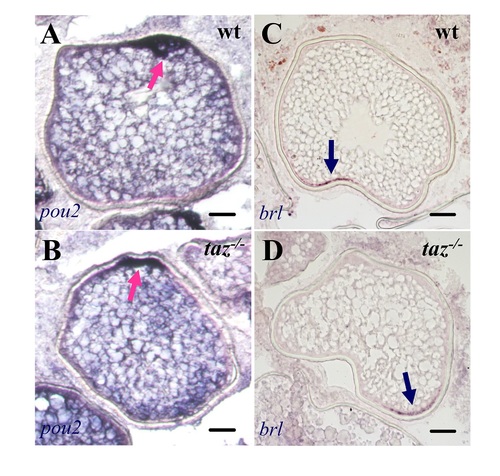
Oocyte polarity is normal in taz-/- zebrafish females.In situ hybridization on sectioned ovaries showed that transcripts of pou2 (A-B) and brl (C-D) were normally located on the animal and vegetal pole, respectively, in both wild type and taz-/-oocytes. Dark blue arrow, vegetal pole; pink arrow, animal pole. Scale bar, 50 μm. |
|
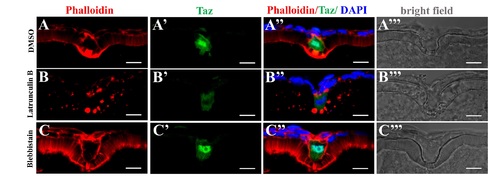
Transient disturbance of actin or Myosin does not significantly affect morphology of the micropylar cell.(A-C’”) Immunofluorescence shows Taz and F-actin in sectioned stage III oocytes after Latrunculin B (B-B’”, n = 7) or Blebbistain (C-C’”, n = 6) treatment. DMSO is the control (A-A’”, n = 7). Transient inhibition of actin polymerization (Latrunculin B) or Myosin II ATPase activity (Blebbistain) does not remarkably affect morphology of the micropylar cell (B”-C”). However, Latrunculin B treatment leads to cytoplasmic retention of Taz in the micropylar cell (6/7, B”), while Blebbistain does not (0/6, C”). Scale bar, 10 μm. |