- Title
-
The Ciliopathy Gene ahi1 Is Required for Zebrafish Cone Photoreceptor Outer Segment Morphogenesis and Survival
- Authors
- Lessieur, E.M., Fogerty, J., Gaivin, R.J., Song, P., Perkins, B.D.
- Source
- Full text @ Invest. Ophthalmol. Vis. Sci.
|
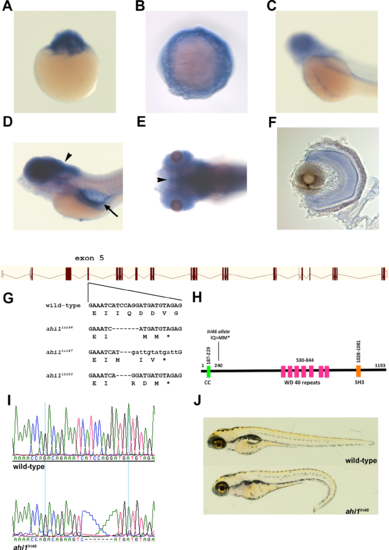
Generation of ahi1 mutant zebrafish. (A?C) Whole-mount in situ hybridization from 1 cell (A), 10 hpf (B), and 48 hpf (C), showing expression of ahi1. (D, E) Lateral and ventral views showing 5 dpf zebrafish stained by whole-mount in situ hybridization. ahi1 expression was observed throughout the central nervous system (arrowheads) and pronephros (arrow). (F) A transverse section shows ahi1 expression throughout the retina at 5 dpf. (G) Illustration of ahi1 genomic architecture. The wild-type sequence and TALENs induced ahi1 mutant alleles are shown. Wild-type nucleotides are in upper case. Deleted nucleotides are denoted as dashes while inserted nucleotides are in lower case letters. (H) Schematic protein structure of Ahi1 illustrating the location of the coiled-coil (CC) domain, WD40 repeats, and SH3 domain. The ahi1lri46 is a frame shift mutation and encodes a truncated protein lacking the 7 WD 40 repeats and the SH3 domain. (I) Chromatograms of Sanger sequencing reactions of ahi1 wild type (wild-type) and homozygous mutant (ahi1lri46) zebrafish. (J) Lateral view of representative wild-type and ahi1lri46 homozygous mutant at 5 dpf. |
|
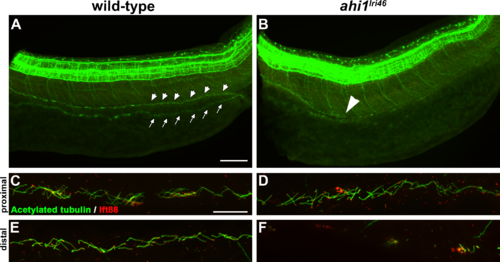
Loss of ahi1 affects other ciliated tissues. (A, B) Anti-acetylated tubulin whole-mount immunostaining (green) of kidney duct in wild-type and ahi1lri46 mutants at 36 hpf. Anterior is to the left in both images. Cilia line the pronephric duct (small arrowheads) in wild-type animals. The contralateral duct also can be observed (small arrows). Cilia are absent in the middle and distal pronephros in ahi1lri46 mutants (arrowhead in [B] indicates the proximal boundary where cilia are missing). (C, D) In the proximal region, mutant cilia are not qualitatively different from wild-type controls. (E, F) Cilia in the distal region of the pronephros are absent in mutant animals when compared to controls. Ift88 immunostaining (red) was used to mark the ciliary transition zone. Scale bars: 50 ?m (A, B) and 20 ?m (C?F). |
|
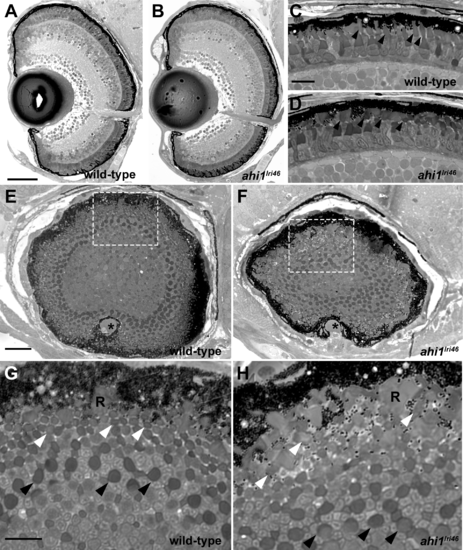
Histologic survey of retinas of 5 dpf wild-type and ahi1lri46 larvae (A?D) Transverse sections of 5 dpf wild-type and ahi1lri46 eyes. Photoreceptor outer segments (arrowheads) were shorter and less organized in ahi1lri46 mutants. (E?H) Sagittal sections of wild-type and ahi1lri46 eyes, taken at the posterior pole just dorsal to the optic nerve (*). Indicated regions (white dashed boxes) in (E, F) are shown at higher magnification in (G, H). Black arrows indicate the larger caliber, more proximal UV cone outer segments. White arrows indicate the smaller caliber, more distal red, green, and blue cone outer segments. R, rod outer segments. Scale bars: 50 ?m (A, B), 10 ?m (C, D, G, H) and 25 ?m (E, F). |
|
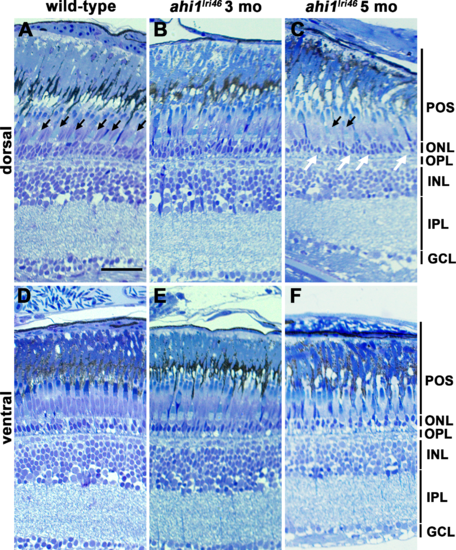
Histologic survey of retinas from wild-type and ahi1lri46 adults. (A?C) Dorsal and (D?F) ventral views of toluidine blue?stained 1-?m thick sections of wild-type, 3- and 5-month-old ahi1lri46 mutants. In the dorsal retina, fewer outer segments of UV-sensitive cones (black arrows) and thinning in the ONL (white arrows) was observed at 5 months of age. POS, photoreceptor outer segments; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar: 50 ?m. PHENOTYPE:
|
|
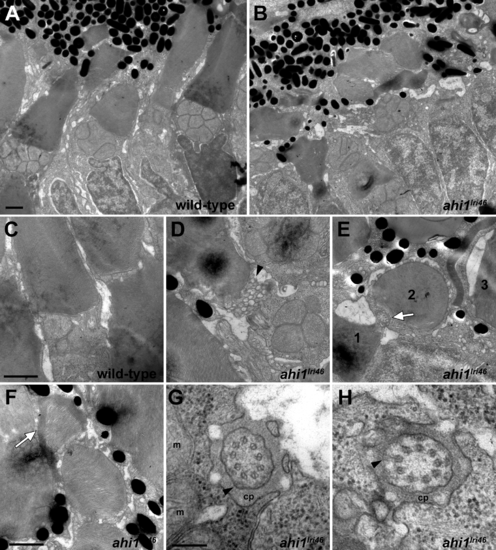
Ultrastructural analysis of wild-type and ahi1lri46 photoreceptors. (A, B) Transmission electron microscopy provided ultrastructural views of wild-type and ahi1lri46 photoreceptors. Mutant outer segments are short, fragmented, and disorganized. (C) Higher magnification of wild-type photoreceptor showing tightly stacked disc membranes and inner segment. (D) Vesiculation at the proximal end of an outer segment in ahi1lri46 mutant (arrowhead). (E) Improper orientation of disc membranes. In this field, an axoneme (white arrow) and disc membranes from outer segments of three different cells, labeled 1 to 3, are oriented perpendicular to each other. (F) Several layers of discs are abnormally wrapped around the outer segment (white arrow). (G, H) Cross-sections through the ciliary pocket (cp) show the proper 9 + 0 microtubule arrangement of axoneme. Mitochondria (m) are visible in (G). Y-shaped crosslinkers also are present (arrows in [G, H]). Staining artifacts are visible as diffuse, black deposits in some panels. Scale bars: 1 ?m (A?F) and 200 nm (G, H). |
|
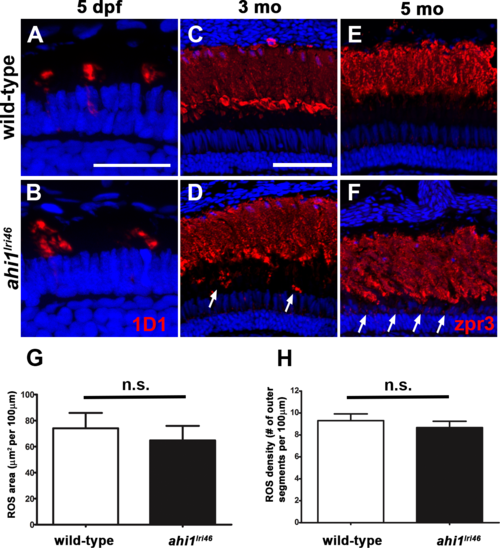
Immunohistochemical analysis of rod photoreceptors in ahi1lri46 mutants. (A, B) Rod photoreceptors stained with 1D1 (red, rhodopsin) at 5 dpf. (C?F) Rod photoreceptors stained with zpr3 (red, rhodopsin) at 3- (C, D) and 5- (E, F) month-old animals. Arrows in (D) and (F) highlight rhodopsin mislocalization. (G) Quantification of rod outer segment area at 5 dpf. (H) Quantification of rod density at 5 dpf. Scale bars: 20 ?m (A?B) and 50 ?m (C?F). |
|
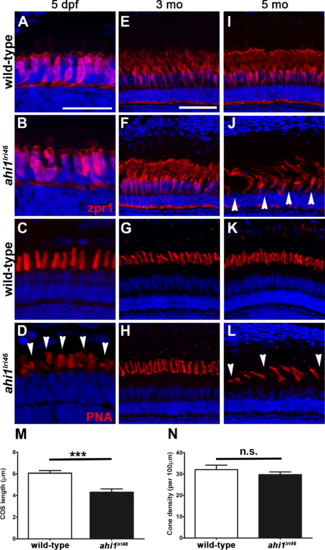
Immunohistochemical analysis of cone photoreceptors in ahi1lri46 mutants. (A, B, E, F, I, J) Red-green double cones are labeled with zpr1 (red). Cone inner segments are preserved at 5 dpf and 3 months of age, but missing (arrowheads) at 5 months. (C, D, G, H, K, L) PNA staining (red) revealed that mutants had shorter cone outer segments ([D], arrowheads) at 5 dpf and missing at 5 months ([L], arrowheads). (M) Quantification of cone outer segment lengths between wild-type and ahi1lri46 mutants at 5 dpf. ***P < 0.001. N, quantification of cone density at 5 dpf. |
|
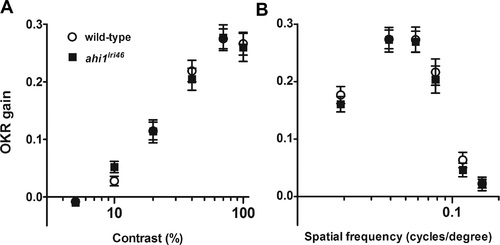
Visual function is not affected in ahi1lri46 mutants at 5 dpf. Optokinetic response of wild-type (open circles) versus ahi1lri46 homozygous mutants (black squares) at 5 dpf as a function of contrast (A) and spatial frequency (B) measured from smooth pursuit eye movements. Contrast sensitivity test n = 21 per genotype. Spatial frequency test n = 14 per genotype. Error bars: SEM. PHENOTYPE:
|
|
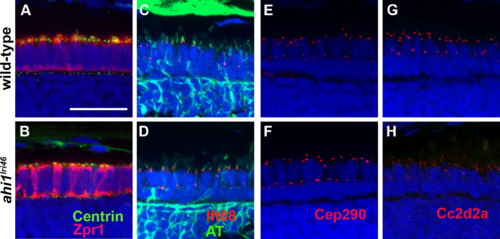
ahi1 is not required for ciliogenesis or transition zone protein localization. (A, B) Tg(centrin:GFP) expression (green) localized to the apical inner segments in wild-type and ahi1lri46 mutants at 3 dpf. Zpr1 (red) was used to stain cone photoreceptors to visualize the apical boundary. (C, D) Five dpf retinas stained with acetylated tubulin (green) and Ift88 (red) showed ciliary localization of the IFT particle in wild-type and mutant animals. (E, H) The transition zone proteins Cep290 and Cc2d2a (red) exhibited punctate staining in wild-type and mutant animals. Scale bar: 20 ?m. EXPRESSION / LABELING:
PHENOTYPE:
|
|
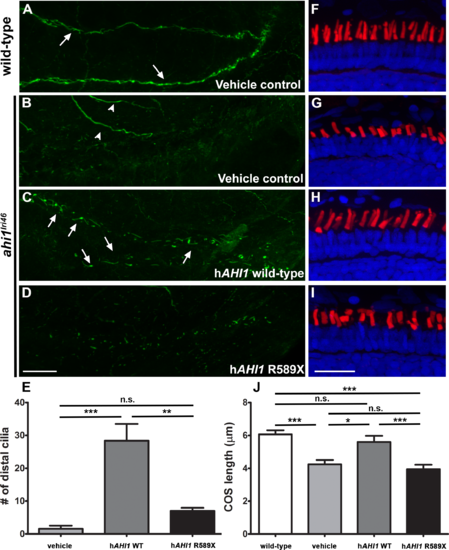
Human AHI1 RNA rescues ahi1lri46 mutant phenotype. (A?D) Whole-mount immunolabeling of pronephric cilia with anti-acetylated tubulin (green) at 36 hpf. Cilia are seen in pronephric ducts of wild-type animals and in ahi1lri46 mutants following injection of mRNA encoding human AHI1 ([A, C]; arrows). Motor neurons also stained with acetylated tubulin ([B], arrowheads). (E) Quantification of cilia numbers in the distal pronephric duct of ahi1lri46 mutants following injection. Distal pronephric cilia in wild-type animals were too numerous to count and are not presented graphically. (F?I) Images of 5 dpf retinas stained with PNA (red) and DAPI (blue) to label cone outer segments. (J) Quantification of cone outer segment lengths (n = 6). Scale bar: 20 ?m. |
|
Zebrafish ahi1 mutants have a characteristic phenotype. Lateral view of 5-month-old wild-type, heterozygous (ahi1lri46/+) and homozygous ahi1lri46 mutant displaying scoliosis of the vertebral column. |