- Title
-
A CRISPR/Cas9 Vector System for Tissue-Specific Gene Disruption in Zebrafish
- Authors
- Ablain, J., Durand, E.M., Yang, S., Zhou, Y., Zon, L.I.
- Source
- Full text @ Dev. Cell
|
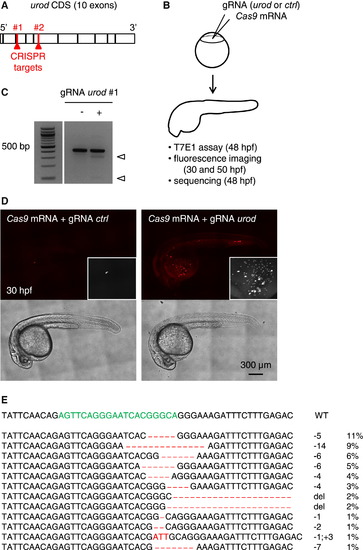
Fluorescent Phenotype Resulting from CRISPR Targeting of urod (A) Schematic representation of urod coding sequence (CDS). The position of the CRISPR target sequences at the beginning of exons three and five are indicated. (B) Outline of the experiment, Cas9 mRNA is injected into one-cell stage embryos along with a gRNA targeting either urod or p53 as a negative control (ctrl). Targeted mutation efficiency is assessed by T7E1 assay and sequencing, and the presence of red fluorescent erythrocytes by confocal microscopy. (C) T7E1 mutagenesis assay at the CRISPR target site in the urod gene. The assay was performed on genomic DNA from 2 dpf embryos injected at the one-cell stage with Cas9 mRNA and either a gRNA against p53 (negative control) or a gRNA against urod. Cleavage bands (arrowheads) indicate the presence of mutations at the target site. See also Figure S1B. (D) Confocal images reveal the presence of fluorescent blood cells in urod mosaic knockout embryos at 30 hpf. The black and white insets show a 2× magnification of the red fluorescent signal in the yolk region. See also Figure S1E. (E) Most frequent (>1%) mutant urod alleles found by deep-sequencing in whole embryos injected with Cas9 mRNA and urod gRNA. The CRISPR target sequence is in green and mutations in red. The type of mutation and the associated frequency (in % of all mutated alleles) are indicated, large deletion (del). |
|
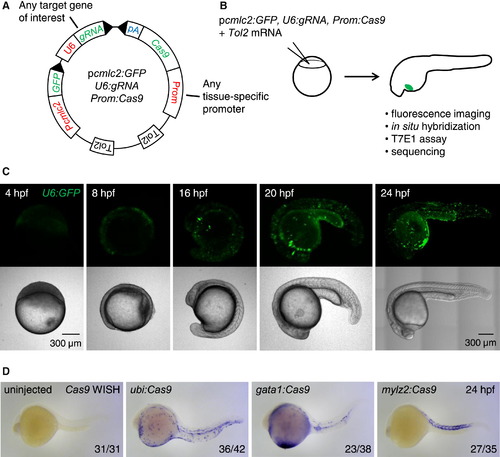
Integratable CRISPR Vector for Tissue-Specific Gene Targeting (A) Schematic representation of the tissue-specific CRISPR vector. Prom denotes any promoter of interest used to drive Cas9 expression in a tissue-restricted manner. GFP expression in the heart of injected embryos is used as a transgenesis marker. Tol2 indicates transposition sites for the Tol2 transposase, SV40 polyA sequence (pA). See Figure S2A for the sequence of U6:gRNA, which comprises two BseRI restriction sites allowing easy cloning of any gene-specific target sequence at the 5′ end of the gRNA. (B) Outline of the experiment, the CRISPR vector is injected into one-cell stage embryos along with Tol2 mRNA. Embryos with GFP-positive hearts are sorted at 24 hpf and further analyzed. Cas9 expression is assessed by in situ hybridization of Cas9 mRNA, targeted mutation efficiency by T7E1 assay and sequencing, and the presence of fluorescent red blood cells by confocal microscopy. (C) Confocal images of embryos injected with Tol2 mRNA and the pcmlc2:GFP, U6:GFP, gata1:Cas9 vector at various time points. Note early (4 hpf) and ubiquitous (24 hpf) GFP expression. (D) Representative images show whole mount in situ hybridization (WISH) using an anti-sense RNA probe against Cas9 mRNA in 24 hpf embryos injected with Tol2 mRNA and pcmlc2:GFP, U6:gRNA urod vectors expressing Cas9 under the control of the indicated promoters. Cas9 expression pattern is governed by the tissue-specificity of the promoters. See also Figure S2B. |
|
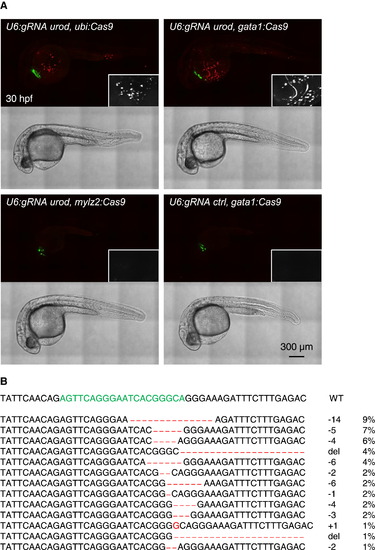
Tissue-Specific urod Inactivation using the CRISPR Vector (A) Confocal images of 30 hpf embryos injected with Tol2 mRNA and the indicated vectors. The fluorescent blood phenotype associated with urod knockout is seen when using the ubiquitous ubi promoter or the erythrocyte-specific gata1 promoter with the gRNA targeting urod, but not when using the muscle-specific mylz2 promoter or a gRNA against p53. See also Figure S3A and Movie S1. (B) Most frequent (>1%) mutant urod alleles found by deep-sequencing in sorted fluorescent red cells from the blood of embryos injected with the pcmlc2:GFP, U6:gRNA urod, gata1:Cas9 vector. The CRISPR target sequence is in green and mutations in red. The type of mutation and the associated frequency (in % of all mutated alleles) are indicated. |
|
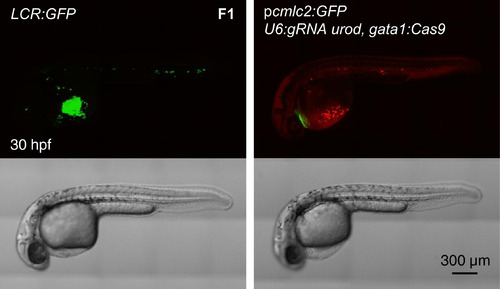
Generation of Transgenic, Tissue-Specific Knockout Fish Confocal images of 30 hpf embryos of the LCR:GFP transgenic line and F1 generation of fish stably expressing pcmlc2:GFP, U6:gRNA urod, gata1:Cas9 vector. See also Figure S4A. |
|
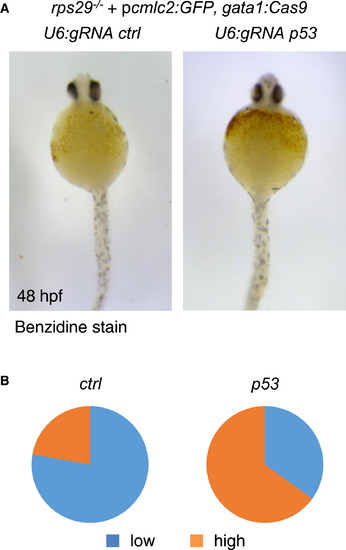
Rescue of an Anemia Phenotype by Erythrocyte-Specific Targeting of p53 (A) Benzidine staining of rps29-/- embryos injected with a pcmlc2:GFP, U6:gRNA, gata1:Cas9 vector targeting urod (ctrl) or p53 at 48 hpf. (B) Quantification of the hemoglobin rescue in rps29-/- embryos. urod (ctrl), n = 18 and p53, n = 20. Pooled results from two independent experiments. |
|
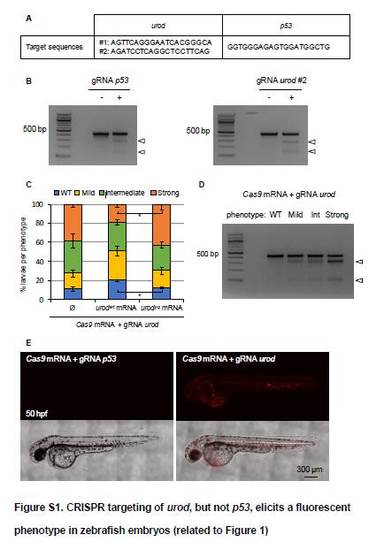
CRISPR targeting of urod, but not p53, elicits a fluorescent phenotype in zebrafish embryos (related to Figure 1) (A) CRISPR target sequences used to knockout the urod and p53 zebrafish genes. (B) T7E1 mutagenesis assay at the p53 CRISPR target site (left) and at the second urod site (right). The assay was performed on genomic DNA from 2 dpf embryos injected at the one-cell stage with Cas9 mRNA and either the gRNA against p53 or the gRNA against urod. Arrows point to cleavage bands. (C) Quantification of the fluorescent phenotype obtained after co-injection of Cas9 mRNA and a gRNA against urod ± wt urod mRNA (urodwt) or yquem urod mRNA (urod mRNA bearing the inactivating mutation found in the yquem mutant, urodyq). WT: no fluorescent blood cell. Mild: <10 fluorescent blood cells. Intermediate: <50 fluorescent blood cells. Strong: >50 fluorescent blood cells. Data represented as mean percentage ± SD of 3 independent experiments. *: p<0.05, paired t-test. (D) T7E1 mutagenesis assay at the CRISPR target site in the urod gene. AB embryos were injected at the one-cell stage with Cas9 mRNA and a gRNA against urod, and sorted into 4 groups (WT, mild, intermediate, high) according to their fluorescent phenotypes (see Figure S1C). The assay was performed on genomic DNA from 8 embryos from each group at 2 dpf. Arrows point to cleavage bands. (E) Confocal images at 50 hpf of embryos injected at the one-cell stage with Cas9 mRNA and either the gRNA against p53 (negative control) or a gRNA against urod. |
|
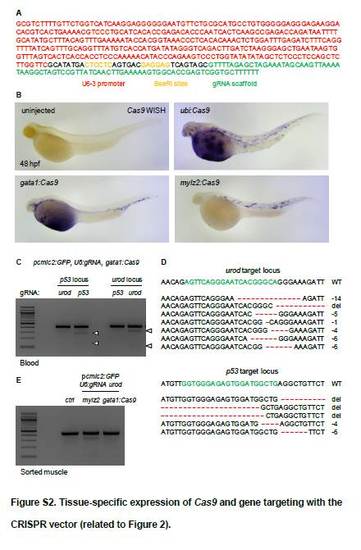
Tissue-specific expression of Cas9 and gene targeting with the CRISPR vector (related to Figure 2). (A) Sequence of the portion of the vector presented in Figure 2A encompassing the U6 promoter and the gRNA scaffold. (B) Representative images of whole mount in situ hybridization (WISH) using an anti-sense RNA probe against Cas9 mRNA in 48 hpf embryos injected with Tol2 mRNA and the pcmlc2:GFP, U6:gRNA vectors expressing Cas9 under the control of the indicated promoters. (C) T7E1 mutagenesis assay at the CRISPR target sites in the p53 or urod genes. The assay was performed on genomic DNA from the blood of 2 dpf embryos injected at the one-cell stage with Tol2 mRNA and the pcmlc2:GFP, U6:gRNA, gata1:Cas9 vectors containing a gRNA against p53 or urod. Arrows point to cleavage bands. (D) Most frequent (>2%) mutant urod and p53 alleles found by deep-sequencing in the blood of embryos injected with Tol2 mRNA and the pcmlc2:GFP, U6:gRNA, gata1:Cas9 vectors containing gRNAs against urod and p53 respectively. The CRISPR target sequence is in green, mutations in red. The type of mutation is indicated (del: large deletion). (E) T7E1 mutagenesis assay at the CRISPR target site in the urod gene. The assay was performed on genomic DNA from sorted mCherry-positive cells from 2 dpf Tg(mylz2:mCherry) embryos injected at the one-cell stage with Tol2 mRNA and the pcmlc2:GFP, U6:gRNA urod, mylz2:Cas9 or pcmlc2:GFP, U6:gRNA urod, gata1:Cas9 vectors. |
|
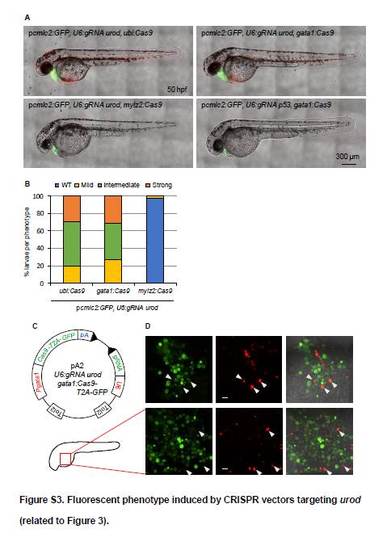
Fluorescent phenotype induced by CRISPR vectors targeting urod (related to Figure 3). (A) Confocal images of 50 hpf embryos injected with Tol2 mRNA and pcmlc2:GFP, U6:gRNA urod vectors expressing Cas9 under the control of the indicated promoters. (B) Quantification of the fluorescent phenotype presented in Figure 3A. WT: no fluorescent blood cell. Mild: <10 fluorescent blood cells. Intermediate: <50 fluorescent blood cells. Strong: >50 fluorescent blood cells. Ubi:Cas9 (n=61), gata1:Cas9 (n=51), mylz2:Cas9 (n=43). (C) Schematic representation of a variant tissue-specific CRISPR vector. Gata1 promoter (Pgata1) drives both Cas9 and GFP expression through a self-cleaving T2A peptide. Any gRNA of interest can be produced ubiquitously off the U6 promoter. Tol2 indicates transposition sites for the Tol2 transposase. pA: SV40 polyA sequence. (D) Confocal images of the yolk region of 30 hpf embryos injected with Tol2 mRNA and the pA2, U6:gRNA urod, gata1:Cas9-T2A-GFP vector. Arrows point to cells that are both green and red. Scale bar: 20 ?m. Note the small size of red-only cells. |
|
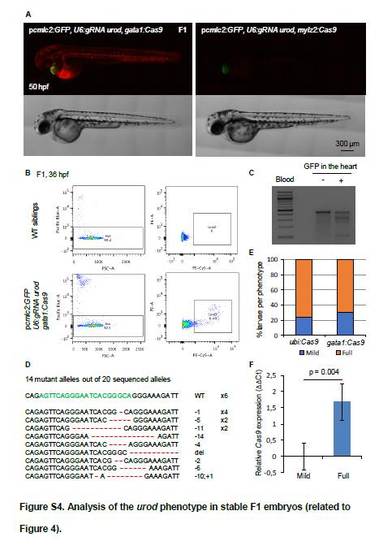
Analysis of the urod phenotype in stable F1 embryos (related to Figure 4). (A) Confocal images of 50 hpf embryos from the F1 generation of fish stably expressing the indicated vectors. (B) FACS analysis of the blood of 36 hpf F1 embryos stably expressing the pcmlc2:GFP, U6:gRNA urod, gata1:Cas9 vector (bottom panels). Blood from F1 siblings not presenting green hearts was used as a negative control (top panels). Red fluorescence due to urod inactivation was detected in the PE-Cy5 channel (right panels). Cytox blue was used to mark dead or dying cells (left panels). (C) T7E1 mutagenesis assay at the CRISPR target site in the urod gene. The assay was performed on genomic DNA from the blood of 2 dpf F1 embryos stably expressing the pcmlc2:GFP, U6:gRNA urod, gata1:Cas9 vector. Blood from F1 siblings not presenting green hearts was used as a negative control. (D) Sequences of the mutant urod alleles found in the blood of embryos stably expressing the pcmlc2:GFP, U6:gRNA urod, gata1:Cas9 vector. The CRISPR target sequence is in green, mutations in red. The type of mutation and the associated number of detected alleles are indicated. (E) Quantification of the fluorescent phenotype observed in F1 embryos. Mild: <10 fluorescent blood cells. Full denotes a number of fluorescent cells comparable to that observed in age-matched LCR:GFP embryos. Ubi:Cas9 (n=37), gata1:Cas9 (n=80). (F) qPCR analysis of Cas9 expression in F1 embryos stably expressing pcmlc2:GFP, U6:gRNA urod, ubi:Cas9 vector. Four embryos showing no or few fluorescent cells (mild) and four embryos showing a full phenotype were compared. Data represented as mean ± SD. |
Reprinted from Developmental Cell, 32(6), Ablain, J., Durand, E.M., Yang, S., Zhou, Y., Zon, L.I., A CRISPR/Cas9 Vector System for Tissue-Specific Gene Disruption in Zebrafish, 756-64, Copyright (2015) with permission from Elsevier. Full text @ Dev. Cell