- Title
-
Mesoderm is required for coordinated cell movements within zebrafish neural plate in vivo
- Authors
- Araya, C., Tawk, M., Girdler, G.C., Costa, M., Carmona-Fontaine, C., and Clarke, J.D.
- Source
- Full text @ Neural Dev.
|
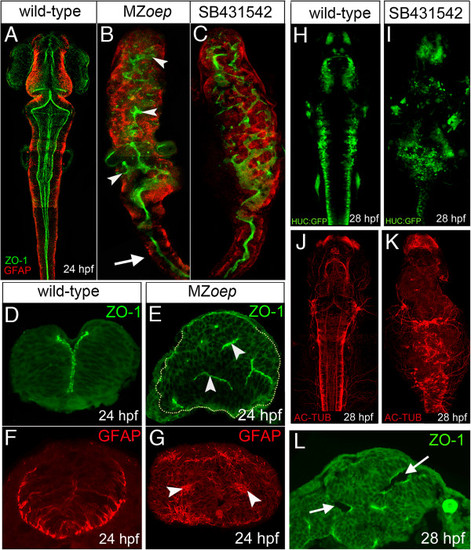
MZoep mutants have aberrant neural tube organization. (A) Projection of confocal z-series showing dorsal view of brain and anterior spinal cord. The midline ventricle is lined by ZO-1 expression (green) and the basal regions of the neuroepithelium are lined by the GFAP expression (red). (B) Projection of confocal z-series showing dorsal view of brain and anterior spinal cord from MZoep mutant. Both apical ZO-1 and basal GFAP expression show extensive disruption to neural tube morphology in brain regions (arrowheads) but appear relatively normal in anterior spinal cord (arrow). (C) Projection of confocal z-series showing dorsal view of brain and anterior spinal cord from embryo treated with the Nodal inhibitor SB-431542. (D,E) Transverse sections show the normal single midline domain of ZO-1 appears discontinuous and more randomly oriented in MZoep embryo. (F,G) The basal marker GFAP is expressed in ectopic foci deep from the surface of the MZoep neural primordium. (H,I,J,K) Neurons labeled with tg(HUC-GFP) (green) and their axons labeled with Ac-tub (red) are present but disorganized in the Nodal-defective embryo brains. (L) By 28 hpf ectopic ventricles (arrowed) have opened up in the MZoep brains. Ac-tub, anti-acetylated tubulin antibody; GFAP, glial fibrillary acidic protein; hpf, hours post fertilization; MZoep, maternal-zygotic one-eyed pinhead; ZO-1, zonula occludens 1. |
|
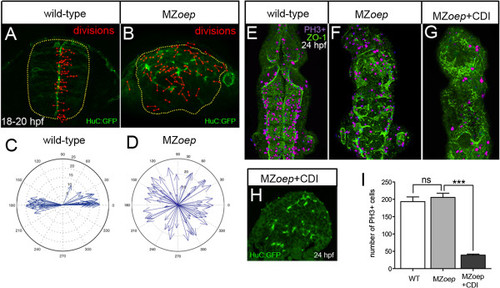
Ectopic divisions do not generate the abnormal neural tube in MZoep embryos. (A) Location and orientation of divisions monitored over a 2-hour time-lapse period of neural rod development in wild-type embryo by analyzing the expression of apical marker Pard3-GFP (green) and H2B-RFP mRNA to label nuclei (not included in image for clarity, red dumbbells indicate location and orientation of dividing cells). Wild-type neural progenitor divisions are strongly orientated along the mediolateral axis of the developing neural tube. Yellow dots outline the rod. (B) Location and orientation of divisions monitored over a 2-hour time-lapse period of neural rod development in MZoep embryo. (C,D) Orientation plots of divisions in wild-type and MZoep embryos. (E,F,G) Dorsal projections of ventricle morphology (ZO-1, green) at 24 hpf in wild-type, MZoep embryos and MZoep embryos treated with CDIs (hydroxyurea and aphidicolin). Blocking division does not rescue ventricle morphology. PH3 staining of mitotic figures (purple) used to calculate efficiency of division block. (H) Transverse section of brain from MZoep embryo treated with division blockers. (I) Quantification of divisions in wild-type, MZoep and MZoep division blocked embryos. Number of cell divisions between wild-type 194 and MZoep 206, P = 0.5129 Student?s t-test and between MZoep 206 and MZoep + CDI 39, ***P <0.0001 Student?s t-test. In (E), (F) and (G), equivalent tissue volumes were used (360 Ám in length x 130 Ám in width x 90 Ám in depth). In (I), error bars indicate SEM. CDI, cell division inhibitor; H2B-RFP, histone H2B/red fluorescent protein fusion; hpf, hours post fertilization; MZoep, maternal-zygotic one-eyed pinhead; Pard3-GFP, green fluorescent protein/polarity protein partitioning defective 3 fusion; PH3, phospho-histone 3 marker; SEM, standard error of the mean; wt, wild-type; ZO-1, zonula occludens 1. EXPRESSION / LABELING:
PHENOTYPE:
|
|
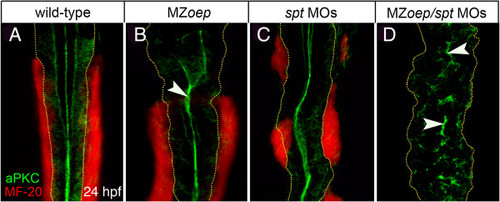
Lack of mesoderm disrupts neural tube morphogenesis in both anterior and posterior levels of the neuro-axis. (A,B) Projection of confocal z-series showing dorsal view of mesoderm (red), expressing the muscle marker (MF-20) and aPKC (green) in caudal hindbrain and anterior spinal cord at 24 hpf. The normal midline domain of aPKC is maintained in regions of MZoep embryo that are adjacent to mesoderm (arrowhead). (C) Mesoderm is still present but disrupted in spt-deficient embryos and the aPKC domain is largely normal. (D) Embryos deficient for both oep and spt have no mesoderm adjacent to the spinal cord and apical aPKC is severely disrupted in spinal cord (arrowheads). Basal extremity of neural tube indicated by yellow dotted lines. aPKC, atypical protein kinase C; hpf, hours post fertilization; MZoep, maternal-zygotic one-eyed pinhead. |
|
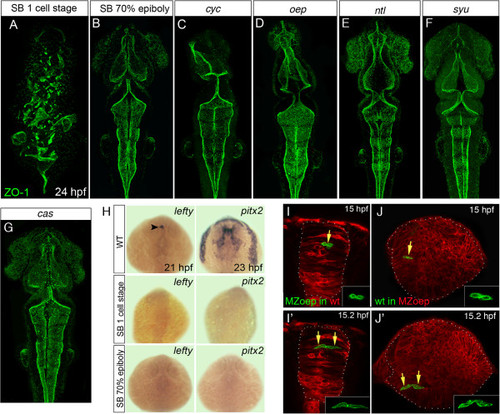
Loss of mesoderm, but not Nodal signaling, leads to neural tube defects. (A,B,C,D,E,F,G) Projection of confocal z-series showing dorsal view of 24 hpf zebrafish embryos staining for the apical marker ZO-1 (green). (A) Disrupted ventricular organization of embryo treated with the Nodal inhibitor SB-431542 from the one-cell stage. (B) Treatment with SB-431542 from 70% epiboly does not cause neural tube defects. (C,D) Ventricle organization in Nodal mutants cyc and oep is largely normal. (E,F) Ventricle organization in ntl and syu mutants is normal. (G) Ventricle organization in the endoderm mutant cas mutants is normal. (H) Expression of the Nodal-dependent markers lefty and pitx2 show the SB-431542 drug is an efficient blocker of Nodal signaling. All embryos between 21 and 23 hpf, dorsal view. Arrowhead in (H) indicates asymmetric lefty expression at 21 hpf. (I,I′) Two frames from time-lapse sequence show transplanted MZoep cells (green) integrate and divide to make mirror-image daughters just like host cells in a wild-type embryo at 15 hpf. (J,J′) Two frames at 15 hpf from time-lapse sequence show wild-type cells (green) divide to make mirror-image daughters after transplantation to a MZoep embryo. Unlike divisions in wild-type embryos, however, these divisions can be far from their normal location at the midline. hpf, hours post fertilization; MZoep, maternal-zygotic one-eyed pinhead; wt, wild-type; ZO-1, zonula occludens 1. EXPRESSION / LABELING:
PHENOTYPE:
|
|
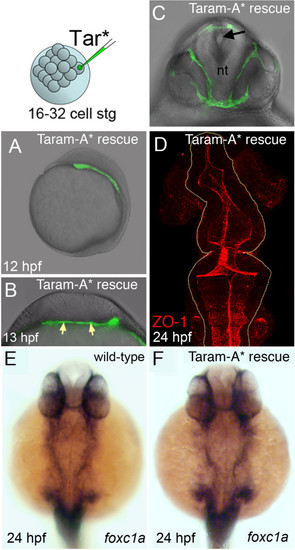
Mesoderm rescues neural tube morphogenesis in MZoep embryos. (A) Lateral view of an MZoep embryo previously injected with the activated form of the TGF-β receptor Taram-A* (Tar*) into a single blastomere. Rescued head mesoderm expresses GFP (green). (B) Transverse section of Taram-A*-injected embryos show rescued mesoderm (green and arrowed) underlying neural plate at 13 hpf. (C) By 24 hpf rescued mesoderm almost surrounds the neural tube (nt) in MZoep embryos. The morphology of the neural tube is rescued and contains a single well-defined midline lumen (black arrow). (D) Rescued neural lumen morphology revealed by ZO-1 expression. (E,F) Identity, distribution and presence of rescued mesoderm, confirmed by the mesendoderm marker foxc1a, in wild-type and Taram-A*-injected MZoep embryos. GFP, green fluorescent protein; hpf, hours post fertilization; MZoep, maternal-zygotic one-eyed pinhead; TGF-β, transforming growth factor beta; ZO-1, zonula occludens 1. |
|
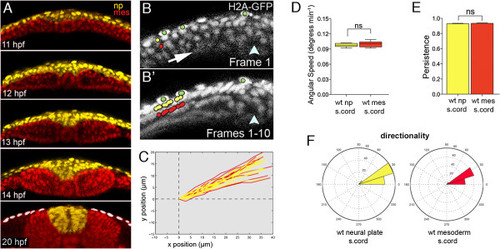
Neural plate and mesoderm move closely together in early stages of zebrafish neurulation. (A) Five frames from a time-lapse movie showing relative organization of mesoderm and neural tissue during neurulation. Mesodermal nuclei are pseudocolored red and neural nuclei are pseudocolored yellow. Tissues remain closely apposed throughout convergence and internalization. White dots indicate the enveloping layer. (B) Frame 1 from a ten-frame time-lapse sequence at level of spinal cord in a wild-type embryo previously injected with H2B-RFP mRNA (gray). Only the left side of the neural plate is shown. Arrow indicates hypothetical tissue movements and arrowhead indicates position of the embryonic midline. Neural cell nuclei marked with yellow dot. Mesoderm cell nuclei marked with red dot. Enveloping layer cell nuclei marked with green dots. (B′) Superimposition of all ten frames from ten-frame time-lapse sequence. The yellow neural nucleus and the red mesodermal nucleus move closely together and in parallel. The green enveloping layer nuclei remain largely immobile. (C) Trajectories of neural (yellow) and mesodermal (red) nuclei in wild-type embryos at spinal cord level. (D) Analysis of angular speeds show neural plate and adjacent mesoderm move with near identical speeds. (E) Analysis of the persistence of movements show neural plate and adjacent mesoderm move with identical persistence. (F) Directionality plots for neural plate and adjacent mesoderm cells are nearly identical. H2B-RFP, histone H2B/red fluorescent protein fusion; wt, wild-type. EXPRESSION / LABELING:
|
|
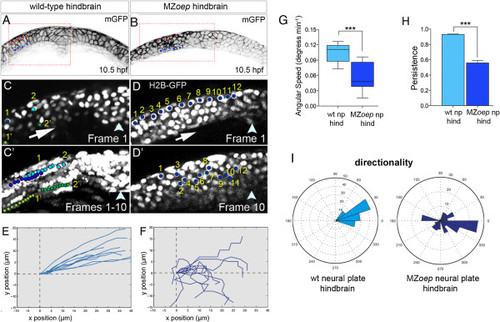
Disrupted cell movements in MZoep neural plate. (A,B) Transverse confocal sections of neural plates at hindbrain level in wild-type and MZoep embryo at 10.5 hpf. Cell outlines revealed by membrane-GFP (black). (C) Frame 1 from a ten-frame time-lapse sequence at level of wild-type hindbrain. Blue nuclei labeled 1 and 2 are in the superficial layer of the neural plate, and green nuclei labeled 1? and 2? are in the deep layer of the neural plate. (C′) Superimposition of all ten frames from ten-frame time-lapse sequence. Superficial blue nuclei remain superficial and deep green nuclei remain deep in the converging neural plate. (D) Frame 1 from a ten-frame time-lapse sequence at level of hindbrain in MZoep embryo. Twelve nuclei in the superficial layer of the neural plate are marked with blue dots. (D′) Position of the 12 nuclei after ten frames shows nuclei are unable to maintain their superficial location in MZoep neural plate. (E) Trajectories of neural nuclei in wild-type embryos at hindbrain level. (F) Trajectories of neural nuclei at hindbrain level in MZoep embryos. (G) Angular speed is significantly reduced in MZoep neural plates (wild-type cells 0.11 degrees/min versus MZoep cells 0.04 degrees/min, ***P <0.0002 Student?s t-test). (H) Persistence is significantly reduced in MZoep neural plates (wild-type cells 0.92 versus MZoep cells 0.56, ***P <0.0001 Student?s t-test). (I) Directionality plots for cells in wild-type and MZoep neural plates. GFP, green fluorescent protein; hpf, hours post fertilization; MZoep, maternal-zygotic one-eyed pinhead; np, neural plate; wt, wild-type. PHENOTYPE:
|
|
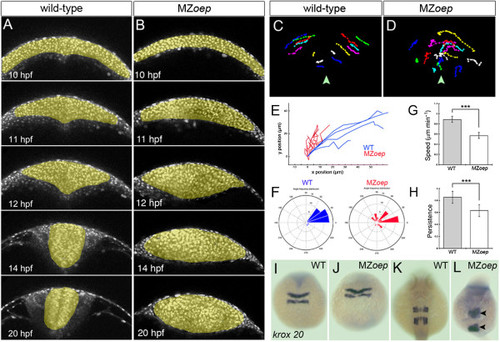
Neural cell movements in MZoep embryos are highly disrupted through neural keel to neural tube stages. (A,B) Selected frames from time-lapse sequences showing normal neurulation in a wild-type embryo and disrupted neurulation in a MZoep embryo from 12 hpf. Cells are labeled with H2B-RFP to reveal movements of nuclei (gray). (C,D) Tracks of individual neural cell nuclei in wild-type and MZoep embryos at hindbrain level. Midline is marked with arrowhead. (E) Trajectory plots of cells in wild-type and MZoep hindbrain primordia. (F) Directionality plots for cells in wild-type and MZoep neural plates. (G,H) Speed and persistence of neural cell movements are disrupted from 12 hpf onwards in MZoep embryos (linear speed Ám/min: wild-type cells 0.87 versus MZoep cells 0.56, ***P <0.0001 Student?s t-test; persistence: wild-type cells 0.85 versus MZoep cells 0.63, ***P <0.0002 Student?s t-test). (I,J,K,L) The hindbrain marker krox20 shows that the anterior-posterior pattern in hindbrain region is only mildly disrupted despite abnormal cell movements in MZoep neural primordia. H2B-RFP, histone H2B/red fluorescent protein fusion; hpf, hours post fertilization; MZoep, maternal-zygotic one-eyed pinhead; wt, wild-type. PHENOTYPE:
|
|
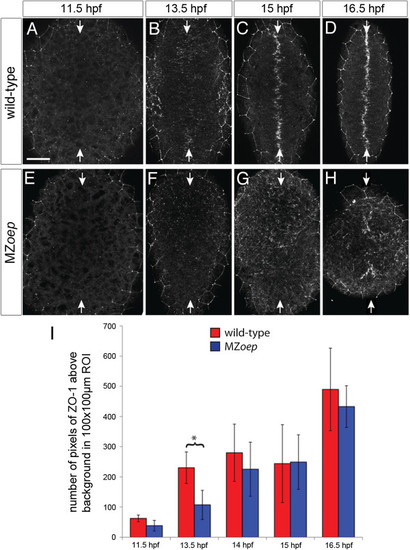
Apical polarity development in MZoep. ZO-1 staining in (A,B,C,D) wild-type and (E,F,G,H) MZoep embryos at different stages of neurulation. All images are maximum confocal projections of six consecutive z-levels taken from a dorsal view at posterior hindbrain and anterior spinal cord regions. Anterior is up. White arrows indicate the dorsal midline of the embryo. The ZO-1 staining surrounding the edge of the neural tissue is from the polarized enveloping layer overlying the neural tissue. Scale bar is 50 Ám. (I) The average number of pixels of ZO-1 staining over time for MZoep and wild-type embryos. At 13.5 hpf the number of ZO-1 puncta in MZoep embryos is significantly different to wild-type (*P <0.01, two-way ANOVA), indicating that ZO-1 polarization is delayed by 30 minutes in MZoep embryos. At subsequent time points (14, 15 and 16 hpf), there is no significant difference between the wild-type and MZoep embryos. Error bars indicate the standard deviation. ANOVA, analysis of variance; hpf, hours post fertilization; MZoep, maternal-zygotic one-eyed pinhead; ZO-1, zonula occludens 1. EXPRESSION / LABELING:
PHENOTYPE:
|