- Title
-
NADPH Oxidase-Driven Phagocyte Recruitment Controls Candida albicans Filamentous Growth and Prevents Mortality
- Authors
- Brothers, K.M., Gratacap, R.L., Barker, S.E., Newman, Z.R., Norum, A., and Wheeler, R.T.
- Source
- Full text @ PLoS Pathog.
|
Photoswitching time-lapse shows phagocytosis block of germination is long-lasting and NADPH oxidase-independent. (A) CAF2-dTomato-infected fish were treated with DMSO or DPI and scored at 4 hpi for fungal morphotype and internalization. Filamentous growth is only seen extracellularly, and this difference is highly significant (p<0.001) in both DMSO (n = 11) and DPI (n = 12), as measured by Fisher′s exact test. Data are pooled from three independent experiments. (B?E) CAF2-dTomato-infected Tg(mpeg1:GAL4/UAS:Kaede) fish were treated with DMSO (n = 11) or DPI (n = 7) from two hours pre-infection and throughout imaging. At 4 hpi, only macrophages at the infection site in the hindbrain were photoswitched green-to-red by exposure to 405 nm laser light. Photoswitched fish were imaged every two hours for an additional 18 hours. (B) Representative images of macrophage movement during the time-lapse, with a schematic above depicting the movement of macrophages out of the hindbrain and acquisition of yellow color as fresh green-fluorescent Kaede protein is produced. Scale bar = 50 μm. (C) Representative images of DMSO- (left) and DPI-treated (right) fish at 18 hpi, showing filamentous growth in the DPI-treated but not the control fish. Scale bar = 50 μm. (D) Macrophages at the site of infection were enumerated at each time-point. Green and red bars represent green-fluorescent (native Kaede) and red or yellow-fluorescent (photoswitched Kaede). Solid bars represent averages from control fish and lightly shaded bars from DPI-treated fish. Although all the infected, DPI-treated, fish died by 18 hpi, uninfected, DPI-treated, fish do not die due to this treatment alone (data not shown and Fig. 5D). (E) Movement of photoswitched macrophages from the hindbrain was tracked for two classes of Kaede+ macrophages: those that had internalized fungi and those that did not phagocytose fungi. A best-fit line for each population shows that half of those that did not phagocytose fungi have left by approximately 18 hpi, whereas there is no appreciable emigration of macrophages with internalized yeast from the hindbrain in this time. (D?E) Shown are the averages per fish pooled from three independent experiments of at least two fish per group. |
|
NADPH oxidase is required for efficient phagocyte recruitment and containment of C. albicans. (A?F) CAF2-dTomato C. albicans or were microinjected into the hindbrain ventricle of (A) Tg(mpx:GFP)i114 (n = 11) or (B) Tg(mpeg1:GAL4/UAS:Kaede) (n = 15) prim25 stage larvae. Fish were treated with DMSO (vehicle) or DPI from two hours prior to infection to 4 hpi and imaged from 0 to 4 hpi by confocal microscopy. (A?B) Images represent at least three independent experiments with three fish of each group per experiment. Scale bars = 10 μm. (C,D) Tg(mpx:GFP)i114 fish with GFP-expressing neutrophils were used. (E?F) Tg(mpeg1:GAL4/UAS:Kaede) fish with Kaede-expressing macrophages were used. (C, E) Phagocytes were counted at the site of infection at 4 hpi. Total phagocytes includes all EGFP+ phagocytes (both with and without yeast) and EGFP phagocytes that internalized fungi. Phagocytes with Candida includes only phagocytes with internalized fungi. Data from at least three independent experiments was pooled and the average and standard error of all fish are shown. (D, F) At 4 hpi, fungi were scored as intracellular or extracellular, and the % internal was calculated per fish. Average and standard error are shown. *p<0.05 **p<0.01. EXPRESSION / LABELING:
|
|
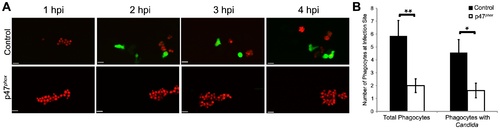
Phox morphants have impaired neutrophil migration. (A?B) CAF2-dTomato C. albicans were microinjected into the hindbrain ventricle of Tg(mpx:GFP)i114 control (n = 7) or p47phox morphants (n = 8) and imaged for 4 hours. (A) Representative time-lapse images of control and p47phox morphants. Results are representative of at least three experiments with at least two fish per group per experiment. Scale bars represent 10 μm. (B) Total phagocytes and phagocytes with internalized fungi were counted at 4 hpi. Numbers from fish over three experiments were pooled and mean and standard error per fish are shown. *p<0.05 **p<0.01 as calculated by two-tailed Student′s T-test. EXPRESSION / LABELING:
|
|
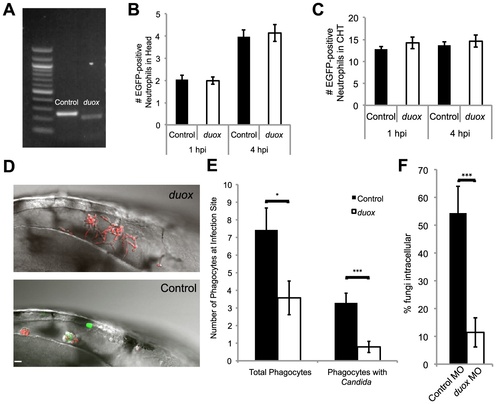
Duox knockdown phenocopies p47phox knockdown and DPI treatment. (A?F) Control and Duox morpholinos were co-injected with p53 morpholino into 1-cell stage Tg(mpx:GFP)i114 zebrafish embryos to create morphants. (A) Samples were collected and prepared for RT-PCR verification of morpholino knockdown. A 39 base pair deletion in the duox message is observed in morphants. (B) Basal level of neutrophils in head at time of infection. Control and duox morphant Tg(mpx:GFP)i114 fish were injected at the prim25 stage with PBS to simulate infection. At 1 hpi and 4 hpi neutrophils in the head were counted at 1 hpi; n = 68 controls and 77 duox morphants, at 4 hpi n = 70 controls and 71 duox morphants. Data pooled from 5 independent experiments. (C) Basal levels of neutrophils in caudal hematopoetic tissue (CHT). Control and duox morphant Tg(mpx:GFP)i114 fish were PBS-injected at the prim25 stage and imaged at 1 hpi and 4 hpi. Data shown are representative of three independent experiments; n = 16 control and n = 13 duox morphants. (D?F) Phagocyte migration. Control (n = 15) and duox (n = 15) morphant Tg(mpx:GFP)i114 fish were injected at the prim25 stage with CAF2-dTomato and imaged until 4 hpi. (D) Representative images of infection site show severe reduction in phagocytosis and extensive extracellular filamentous growth in duox morphants (top) compared with controls (bottom). Scale bar = 10 μm. Representative of three independent experiments. (E) Phagocytes were counted at the site of infection at 4 hpi. Total phagocytes includes all EGFP+ neutrophils and EGFP- phagocytes that internalized fungi. Phagocytes with Candida includes only phagocytes with internalized fungi. Data from all experiments was pooled and the average and standard error of all fish are shown. (F) At 4 hpi, fungi were scored as intracellular or extracellular, and the percent internal was calculated per fish. Data from three independent experiments were pooled and the average per fish and standard error are shown. *p<0.05 **p<0.01 ***p<0.001. |
|
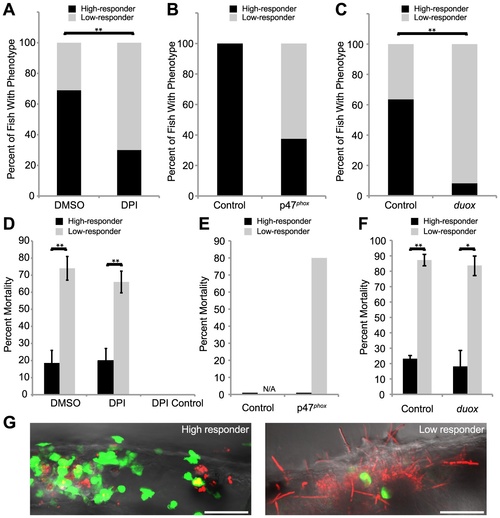
Weak early immune response permits filamentous growth and promotes pathogenesis. (A?F) Tg(mpx:GFP)i114 fish with green fluorescent neutrophils were infected with CAF2-dTomato at the prim25 stage, imaged at 4 hpi by microscopy to quantify phagocytosis efficiency, then sorted into individual wells in a 24-well dish and scored for survival at 24 hpi. (A?C) Low and high responders were quantified at 4 hpi by microscopy. Low responders had five or greater extracellular fungi at 4 hpi. (A,C) Data shown are means from three independent experiments that were analyzed by two-tailed Student′s T-test. (B, E) Data shown are pooled from three independent experiments, as the number of total fish was too small (n = 6) for similar statistical analysis. (D?F) The percent of high or low responders that died by 24 hpi was quantified. Data shown are either the averages and standard errors (D and F) or were pooled (E) from three independent experiments. Statistical analysis was performed by Student′s T-test (D and F). (A, D) Infected fish were treated with DMSO (n = 131) or DPI (n = 118) from 2 hours pre-infection to 4 hpi. (B, E) Control (n = 7) or p47phox (n = 8) morphants were infected and followed. (C,F) Control (n = 129) or duox (n = 139) morphants were infected and followed. (G) High responders (left) contain the infection by 24 hpi, in contrast to low responders (right) which permit filamentous growth and more frequently die by 24 hpi. Images are representative of three independent experiments. Scale bar = 50 μm. N/A = not applicable because no fish in this category. *p<0.05, **p<0.01. PHENOTYPE:
|
|
The EDT1-dependent morphogenetic switching pathway plays roles in NADPH oxidase-independent phagocyte migration and virulence. (A?E) prim25 stage Tg(mpx:GFP)i114 larvae were injected with CAF2-dTomato, edt1?/?-dTomato, or edt1?/EDT1-dTomato C. albicans. Fish were incubated with DMSO or DPI from two hours pre-infection until 4 hpi, and imaged by confocal microscopy. At 4 hpi phagocyte migration and phagocytosis were quantified and fish were sorted into high- and low-responder categories, and at 24 hpi fish were scored for survival. (A) Images are representative of three independent experiments; n = 6 for each fungal genotype and treatment group. Time-lapse images from edt1?/EDT1 infections are indistinguishable from wildtype infections but are not shown due to space considerations. Scale bar = 50 μm. (B) Quantitation of total phagocyte response and number of phagocytes with internalized fungi shows the average and standard error from fish pooled from three independent experiments; n = 6 for each fungal genotype and treatment group. (C) Phagocytosis efficiency was measured in fish pooled from three independent experiments; n = 6 for each fungal genotype and treatment group. (D) Survival percentage of low- and high-responders was measured for each of three independent experiments, and the means are shown; n = 70?74 for each group. (E) Low mortality of low-responders infected with yeast-locked edt1?/? mutant. Means and standard errors of mortality percentages of low- and high-responders at 24 hpi are shown. (B?E) Data represent three independent experiments. Means and standard errors are shown. Differences between groups were assessed by two-tailed Student′s T-test (B, C, and E) or Fisher′s exact test (D). *p<0.05, **p<0.01, ***p<0.001, n.s. no significant difference. EXPRESSION / LABELING:
|
|
C. albicans does not induce detectable respiratory burst activity in recruited phagocytes. AB zebrafish were raised to prim25 stage were either injected in the hindbrain (A, B) or not injected (C). Those injected were inoculated with 3 nL of (A) PBS+1.5×107 cfu/mL CAF2-dTomato or (B) PBS. (D) As a positive control for uncaging of H2DCF-DA by reactive oxygen species, some fish were injected in the otic vesicle with 3 nL PBS+0.5% H2O2. Each group was divided into two and incubated in H2DCF-DA (500 ng/mL in 0.2% DMSO; left panels) or vehicle control (0.2% DMSO; right panels) for 1 hour. Ten fish per group were screened between one and two hours post-injection and images were acquired from representative animals. Composites include maximum projections of the red and green channels (25 slices) overlaid with a single slice in the DIC channel, or maximum projections in the red and green channels only. All images were acquired with the same settings to compare them, ensuring that the green channel detector was set just below saturation for the otic vesicle fluorescence in PBS+H2O2 injected fish. Enhancing the gain to image background fluorescence did not reveal any additional phagocytes with increased green fluorescence. In 20 fish, imaged from two independent experiments, zero cells were observed producing a detectable green fluorescence above the background level when injected with C. albicans. Further imaging with dihydrorhodamine gave similarly negative results in two independent experiments (data not shown). Scale bars represent 100 μm. |
|
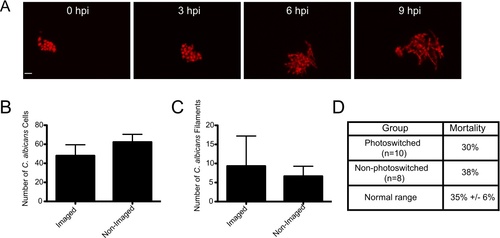
Confocal imaging does not notably inhibit fungal growth, filamentation, or virulence. (A?C) CAF2-dTomato C. albicans were microinjected into the hindbrain ventricle of AB zebrafish and fish were screened for inoculum. One group of fish was imaged from 0 to 9 hpi, while the other was imaged only at 9 hpi. Imaging performed with 543 nm He-Ne laser at 88% power, using a 40× 0.75NA objective, with slices at 1.5 μm spacing. Pixel dwell time was 4 μs, each image dimension was 1024×1024, and total number of slices ranged from 32?40, with total active imaging time of 5?7 minutes per stack and 45?63 minutes for each fish total over the course of the experiment. (A) Image panels of a fish imaged from 0 to 9 hpi with florid fungal filamentation. Scale bar = 10 μm. (B) The number of C. albicans cells per fish was quantified at 9 hpi for each imaging group (N = 3) with no statistical significance (p>0.05). (C) The number of C. albicans filaments per fish were quantified at 9 hpi for each imaging group (n = 3) with no statistical significance (p>0.05). Error bars represent standard error of the mean. These results are representative of a large number of experiments that have been performed but were not specifically quantified. (D) Comparison of mortality in photoswitching experiments. Fish were infected and imaged as described for Fig. 1. Photoswitching was carried out as described in the Materials and Methods. Mortality was assayed at 24 hpi for photoswitched Tg(mpeg1:GAL4/UAS:Kaede) fish (N = 12) and non-photoswitched Tg(mpeg1:GAL4/UAS:Kaede) fish (N = 8). This is compared to typical 24 hpi mortality (35%) seen in this infection model for control fish in experiments described in Fig. 5 and Fig. 6 (n = 8 independent experiments). |
|
In the swimbladder, DPI reduces the neutrophil migration to wounding, but not to C. albicans. (A) Diagram illustrating the injection site (red line) in 4 dpf zebrafish. (B and C) 4 dpf mpx:GFP zebrafish were treated from 1 hour pre-injection to 4 hpi with 100 μM DPI (in DMSO) or vehicle (0.8% DMSO). Fish were injected with 5?20 yeasts of C. albicans CAF2-dTomato in the swimbladder. (B) The C. albicans inoculum was quantified by confocal microscopy within 30 minutes post-infection. (C) Neutrophils were enumerated by confocal microscopy, at the site of infection (SOI) 4 hours post infection. Four independent experiments were performed, with comparable results, and individuals from all four experiments are pooled together. Means and standard error of the mean are represented. N = 33, 30, 62, 46, 67 and 35 for the following six conditions: No injection (DMSO treated and DPI treated); PBS injection (DMSO treated and DPI treated); and Caf2-dTomato injection (DMSO treated and DPI treated), respectively. Statistical tests performed were Mann-Whitney (for panel A) and Kruskall-Wallis (for panel B), n.s. non significant, * p<0.05. (D) Representative images of 4 dpf mpx:GFP zebrafish 4 hpi. Images are composites of maximum projection of the red and green channels (20 slices for no injection and PBS injection and 25 slices for CAF2-dTomato injection) overlaid with a single slice in the DIC channel. Blue outlines in images define the swimbladder site of injection where the neutrophils were counted. Scale bars = 100 μm. |