- Title
-
Regulation of endoderm formation and left-right asymmetry by miR-92 during early zebrafish development
- Authors
- Li, N., Wei, C., Olena, A.F., and Patton, J.G.
- Source
- Full text @ Development
|
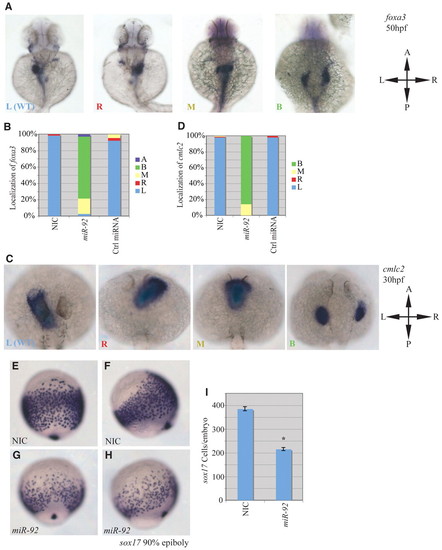
miR-92 gain of function. Gain-of-function experiments were performed by injection of single-cell zebrafish embryos with miR-92 followed by localization of specific markers, as indicated. (A) Localization of foxa3 in wild-type embryos and those injected with miR-92 at 50 hpf. Views are dorsal with anterior to the top. Images are of representative embryos with liver primordia localized to either the left (L), right (R), midline (M) or bilateral (B) positions. (B) Percentages of left, right, midline or bilateral localization of foxa3 in non-injected control (NIC) (n=188), miR-92-injected (n=37) and control miRNA-injected (n=90) embryos. In rare cases, no expression of foxa3 was detected (A, absent). (C) Localization of cmlc2 in wild type and in embryos injected with miR-92 at 30 hpf. Views are dorsal with anterior to the top. Images are of representative embryos with cardiac primordia localized to the left, right, midline or bilateral positions. (D) Percentages of left, right, midline and bilateral localization of cmcl2 in NIC (n=103), miR-92-injected (n=98) and control miRNA-injected (n=53) embryos. (E-H) Localization of sox17-expressing cells in wild-type embryos and miR-92-overexpressing embryos at 90% epiboly. (E,G) Dorsal views with anterior to the top. (F,H) Lateral views with dorsal to the right. (I) Numbers of sox17-expressing cells in NIC (n=16) and miR-92-injected embryos (n=11). Error bars represent s.e.m. *, P<0.01; Student′s t-test. A, anterior; P, posterior; L, left; R, right. |
|
miR-92 loss of function. Loss-of-function experiments were performed by injection of antisense MOs against miR-92 into single-cell zebrafish embryos followed by localization of markers, as indicated. (A) Percentages of left (L), right (R) and midline (M) localized foxa3 in NIC (n=188), miR-92 MO1-injected (n=55), miR-92 MO2-injected (n=15), MO1+2-injected (n=126) and control MO-injected (n=56) embryos. (B) Percentages of left, right and midline localized cmcl2 in NIC (n=103), MO1-injected (n=102), MO2-injected (n=97), MO1+2-injected (n=62) and control MO-injected (n=53) embryos. (C-F) Confocal z-stacks of Kupffer′s vesicle (KV) in NICs, MO1-injected and MO2-injected embryos and in miR-92 morphants co-injected with miR-92 RNA into the dorsal forerunner cells (DFCs) (Rescue) at the10-somite stage using a sox17:gfp transgenic line that labels KV cells green. Motile cilia were identified by immunohistochemistry with antibodies against acetylated tubulin (red). Scale bars: 5 μm. (G) GFP-positive KV cells were counted and cilia length measured in the indicated embryos. Error bars represent s.e.m. *, P<0.01 for KV cell number between miR-92 morphants and NICs, P<0.05 for cilia length and P<0.05 for KV cell number between miR-92 morphants and rescue embryos; Student′s t-test. MO refers to miR-92 MO. (H-K) Localization of sox17-expressing cells in wild-type embryos (NIC) and miR-92 morphants (MO) at 90% epiboly. (H,J) Dorsal views with anterior to the top. (I,K) Lateral views with dorsal to the right. (L) Numbers of sox17-expressing cells in NICs (n=16) and miR-92 morphants (MO; n=13). Error bars represent s.e.m. *, P<0.01; Student′s t-test. EXPRESSION / LABELING:
PHENOTYPE:
|
|
gata5 is a target of miR-92. (A) The GFP reporter fused to the 32UTR of gata5. Base pairing between miR-92 and two miRNA recognition elements (MREs) is shown. (B-G) Single-cell zebrafish embryos were injected with mRNAs encoding GFP reporters and fluorescence was monitored at 1 dpf. Embryos were injected with reporters containing either the full-length gata5 32UTR (full) or a construct in which both MREs were deleted (D12). MO refers to miR-92 MO. (H) Western blot of lysates from embryos injected as above were performed with antibodies against GFP or α-tubulin as a loading control. (I) Quantification of relative GFP expression. Error bars represent s.e.m. *, P<0.01, between indicated injection and control embryos (n>3); Student′s t-test. |
|
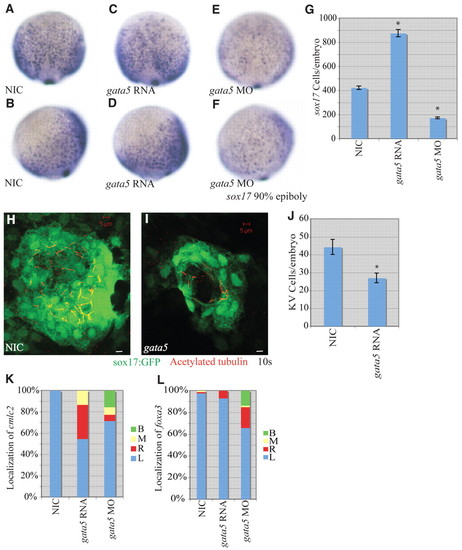
gata5 effects on early zebrafish development. (A-F) Localization of sox17-expressing cells in wild-type, gata5 gain-of-function and gata5 loss-of-function embryos at 90% epiboly. (A,C,E) Dorsal views with anterior to the top. (B,D,F) Lateral views with dorsal to the right. (G) Numbers of sox17-expressing cells in NICs (n=4), gata5 gain-of-function (n=7) and gata5 loss-of-function (n=8) embryos. Error bars represent s.e.m. *, P<0.01 between injected embryos and NIC; Student′s t-test. (H,I) Confocal z-stacks of KV in NIC and gata5-injected embryos at the 10-somite stage. KVs were labeled using a sox17:gfp (green) transgenic line as above. Motile cilia were labeled with antibodies against acetylated tubulin (red). Scale bars: 5 μm. (J) KV cell number was counted as above. Error bars represent s.e.m. *, P<0.01 between NIC and gata5 gain of function; Student′s t-test. (K) Percentages of left (L), right (R), midline (M) and bilateral (B) localized cmcl2 in NIC (n=49), gata5 gain-of-function (n=75) and gata5 loss-of-function (n=84) embryos. (L) Percentages of left, right, midline and bilateral localized foxa3 in NIC (n=83), gata5 gain-of-function (n=142) and gata5 loss-of-function (n=73) embryos. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Epistatic interaction between miR-92 and gata5. (A-E) Localization of sox17-expressing cells in wild-type and variously injected zebrafish embryos at 90% epiboly. MO refers to miR-92 MO. Views are lateral with dorsal to the right. (F) Numbers of sox17-expressing cells in NICs (n=4), miR-92 gain-of-function (n=4), miR-92 and gata5 co-injected (n=7), miR-92 loss-of-function (n=4) and miR-92 MO and gata5 MO co-injected (n=7) embryos. Error bars represent s.e.m. *, P<0.01 between injected embryos and NIC; Student′s t-test. (G) Percentages of left (L), right (R), midline (M) and bilateral (B) localized cmlc2 in NIC (n=112), miR-92 gain-of-function (n=67) and miR-92 and gata5 co-injected (n=31) embryos. (H) Percentages of left, right, midline and bilateral localized cmlc2 in NIC (n=97), miR-92 loss-of-function (n=61) and miR-92 MO and gata5 MO co-injected (n=81) embryos. |
|
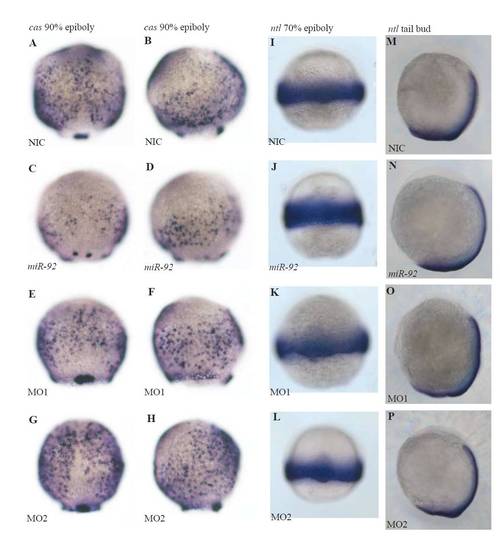
Expression of casanova and no tail transcripts in wild-type, miR-92 gain-of-function and miR-92 loss-of-function embryos. (A-H) cas (sox32) expression is downregulated by miR-92 gain of function and upregulated by miR-92 loss of function. (I-P) ntl expression is not significantly altered by misregulation of miR-92. (A,C,E,G,I-L) Dorsal views with anterior to the top. (B,D,F,H,M-P) Lateral views with embryonic dorsal to the right. |
|
Knockdown of miR-92. Northern blot of miR-92 levels in the absence (NIC) or presence of combinations of morpholinos targeting miR-92. U6 provides a loading control. |
|
Ciliogenesis is not disrupted in miR-92 morphants. (A,B) Expression of lrdr in wild-type and MO-injected embryos at 90% epiboly. (C,D) Expression of foxj1 in wild-type and MO-injected embryos at 100% epiboly. (E,F) Expression of fgf8 in wild-type and MO-injected embryos at the 6-somite stage. (G-J) Motile cilia in the inner ear are intact in wild-type, miR-92 gain-of-function and miR-92 loss-of-function embryos at 1 dpf. (K-N) Motile cilia in the pronephros are intact in wild-type, miR-92 gain-of-function and miR-92 loss-of-function embryos at 1 dpf. Red, cilia staining with antibodies against acetylated tubulin. Blue, SYTO59 staining of nuclei. |