- Title
-
Generation of a non-leaky heat shock-inducible Cre line for conditional Cre/lox strategies in zebrafish
- Authors
- Hans, S., Freudenreich, D., Geffarth, M., Kaslin, J., Machate, A., and Brand, M.
- Source
- Full text @ Dev. Dyn.
|
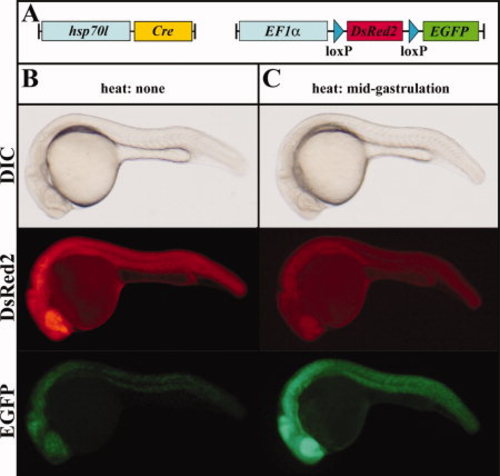
Cre-mediated recombination of the Tg(hsp70l:Cre) allele in the red-to-green reporter line in the absence and presence of heat. A: Scheme of the transgene combination of the embryos depicted in B and C. B: In the absence of heat, the combination of the Tg(hsp70l:Cre) allele with the red-to-green reporter line results in strong DsRed2 and mosaic EGFP expression in double transgenic embryos. C:In contrast, brief heat induction at mid-gastrulation stages results in reduced DsRed2 and strong ubiquitous EGFP expression in double transgenic embryos. B,C: Lateral views of live 24-hpf embryos. |
|
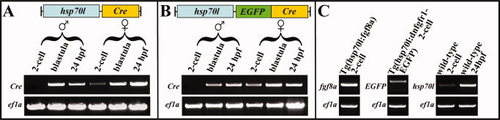
RT-PCR reaction analysis of the Tg(hsp70l:Cre), the Tg(hsp70l:EGFP-Cre), the Tg(hsp70l:fgf8a), and the Tg(hsp70l:dnfgfr1-EGFP) alleles as well as the endogenous hsp70l gene at permissive temperatures. A: When the Tg(hsp70l:Cre) allele is provided paternally, no Cre transcripts can be detected at the 2-cell stage but at late blastula stages and 24 hpf. In contrast, maternal contribution of the Tg(hsp70l:Cre) allele results in the detection of Cre transcripts at all examined stages. B: Similarly, if the Tg(hsp70l:EGFP-Cre) allele is provided paternally, Cre transcript can not be detected at the 2-cell stage but at late blastula stages and 24 hpf, whereas maternal contribution of the Tg(hsp70l:Cre) allele is present at all stages. C: Maternal contribution of the Tg(hsp70l:fgf8a) and the Tg(hsp70l:dnfgfr1-EGFP) alleles result in the detection of fgf8a and EGFP transcripts at the 2-cell stage. In wild-type embryos, hsp70l can be detected at the 2-cell stage and at 24 hpf. |
|
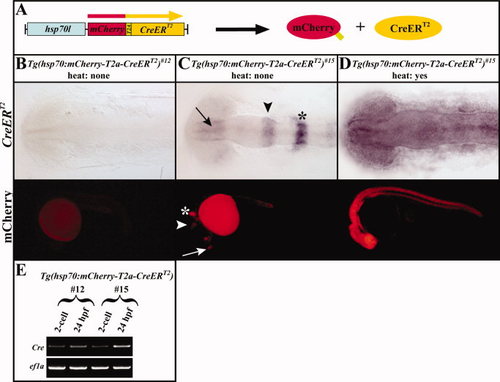
Regulation of mCherry and CreERT2 expression in the absence and presence of heat. A: Scheme of the Tg(hsp70l:mCherry-T2A-CreERT2) construct, which expresses a single open reading frame coding for mCherry (red) and CreERT2 (yellow) separated by a viral T2A peptide sequence (green) under the control of a zebrafish heat shock?inducible hsp70l promoter (blue). After translation, the viral T2A peptide cleavage leads to the production of non-fused mCherry and CreERT2 proteins. B: In the absence of heat, the Tg(hsp70l:mCherry-T2A-CreERT2)#12 allele is transcriptionally silent on CreERT2 RNA and fluorescent mCherry level, respectively. C: In contrast, the Tg(hsp70l:mCherry-T2A-CreERT2)#15 enhancer trap allele is expressed in the forebrain (arrow), midbrain-hindbrain boundary (arrowhead), and rhombomere 4 of the hindbrain anlage (asterisk) at permissive temperatures. D: After heat shock, CreERT2 RNA and fluorescent mCherry are expressed in a ubiquitous fashion. E: RT-PCR reaction analysis of the Tg(hsp70l:mCherry-T2A-CreERT2) alleles. When the Tg(hsp70l:mCherry-T2A-CreERT2) allele is provided maternally, Cre transcripts are detected for both alleles at the 2-cell stage and 24hpf. B?D: Dorsal and lateral views of transgenic embryos at the 12-somite stage and live 24-hpf embryos, respectively. |
|
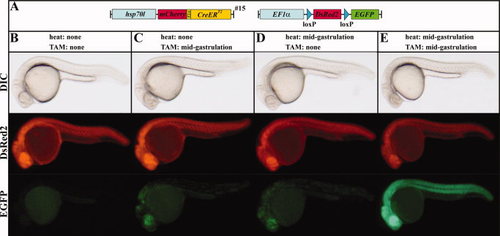
Cre-mediated recombination of the (hsp70l:mCherry-T2A-CreERT2)#152 allele in the red-to-green reporter line in the absence and presence of heat and TAM. A: Scheme of the transgene combination of the embryos depicted in B?E. B: In the absence of heat and TAM, the combination of the Tg(hsp70l:mCherry-T2A-CreERT2)#15 allele with the red-to-green reporter line results in strong DsRed2 but no EGFP expression in double transgenic embryos. C: Application of TAM leads to EGFP expression in the Tg(hsp70l:mCherry-T2A-CreERT2)#15 enhancer trap as well as a mosaic pattern in double transgenic embryos at permissive temperatures. D: In the absence of TAM, mosaic EGFP expression can be detected after heat treatment. E: Application of TAM and heat results in strong ubiquitous EGFP expression in double transgenic embryos. B?E: Lateral views of live 24-hpf embryos. |
|
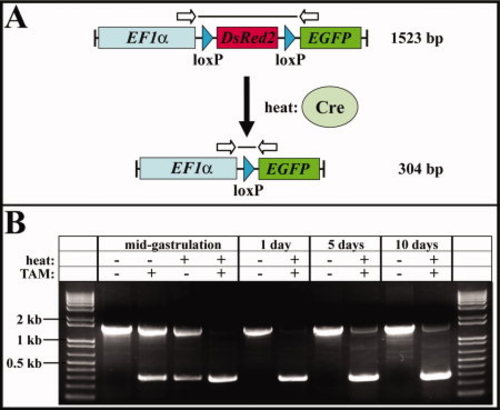
Detection of Cre-mediated site-specific deletion by PCR analysis. A: Scheme of the expected PCR products before (1,523 bp) and after (304 bp) Cre-mediated recombination in the red-to-green reporter line using the primers indicated as arrows. B: Cre-mediated recombination of the (hsp70l:mCherry-T2A-CreERT2)#15 allele in the red-to-green reporter line in the absence (-) or presence (+) of heat and/or TAM at different developmental stages (mid-gastrulation, 1 day, 5 days, 10 days). In the absence of heat and TAM, only the unrecombined product of 1,523 bp can be detected, whereas application of heat or TAM or both leads to the amplification of the recombined 304-bp fragment. kb, kilobase. |
|
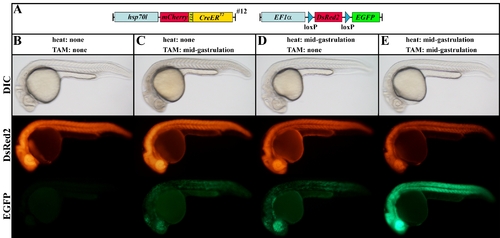
Cre-mediated recombination of the (hsp70l:mCherry-T2A-CreERT2)#12 allele in the red-to-green reporter line in the absence and presence of heat and TAM. A: Scheme of the transgene combination of the embryos depicted in B?E. B: In the absence of heat and TAM, the combination of the Tg(hsp70l:mCherry-T2A-CreERT2)#12 allele with the red-to-green reporter line results in strong DsRed2 but no EGFP expression in double transgenic embryos. C: Application of TAM leads to mosaic EGFP expression in double transgenic embryos at permissive temperatures. D: In the absence of TAM, mosaic EGFP expression can be detected after heat treatment. E: Application of TAM and heat results in strong ubiquitous EGFP expression in double transgenic embryos. B?E: Lateral views of live 24-hpf embryos. |
|
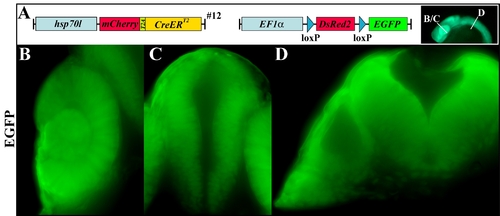
Recombination efficiency of the (hsp70l:mCherry-T2A-CreERT2)#12. A: Scheme of the transgene combination and the dissection plane of the embryos depicted in B?D. Cross-sections of double transgenic embryos examined by immunohistochemistry against EGFP showing uniform EGFP expression in the retina (B), diencephalon (C), and hindbrain (D). |
|
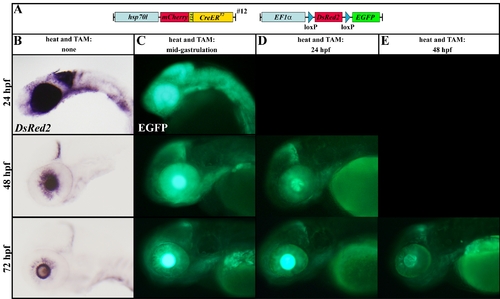
Recombination of the (hsp70l:mCherry-T2A-CreERT2)#12 allele at later stages. A: Scheme of the transgene combination of the embryos depicted in C?E. B: Expression of DsRed2 in the red-to-green reporter line at 24, 48, and 72 hpf revealed by in situ hybridization. Fluorescent EGFP expression profile in double transgenic embryos after heat induction and TAM treatment at mid-gastrulation stages (C), 24 hpf (D), and 48 hpf (E), respectively. |