- Title
-
Analysis of heart valve development in larval zebrafish
- Authors
- Martin, R.T., and Bartman, T.
- Source
- Full text @ Dev. Dyn.
|
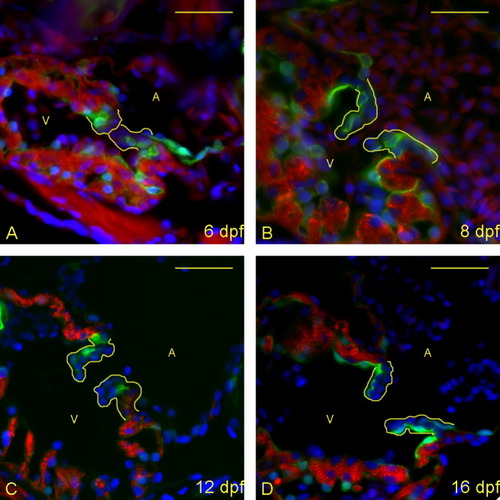
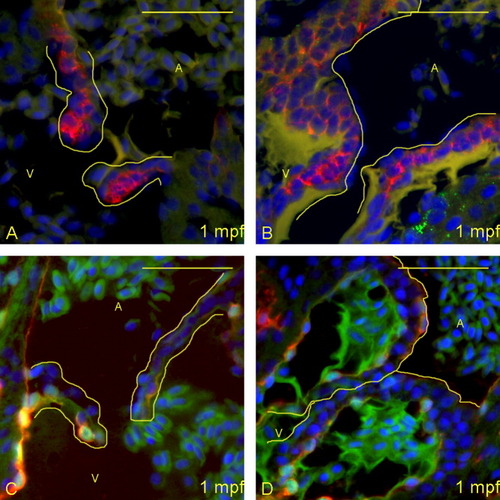
Zebrafish heart valves elongate but remain highly cellular between 6 and 16 days postfertilization (dpf). A-D: Five micrometer paraffin sections of flk1::GFP (GFL, green fluorescent protein) transgene-carrying zebrafish embryos/larvae were imaged to identify the morphology of the atrioventricular (AV) boundary and intraluminal structure. Atria (A) and ventricles (V) are labeled (yellow letters), and yellow lines outline the extent of the AV structure (cushion and/or valve). Staining identifies cell nuclei (DAPI, 4′,6-diamidine-2-phenylidole-dihydrochloride; blue), myocardium (anti-MF-20: red), and endothelial cells (anti-GFP; green). Through this time period, the valves progressively lengthen and thin, and little to no extracellular matrix is seen within the valve leaflets. The most significant change is in cellular organization from 12 (C) to 16 (D) dpf, with the leaflets developing a linear, two-cell-layer thick form. Scale bar = 20 μm. |
|
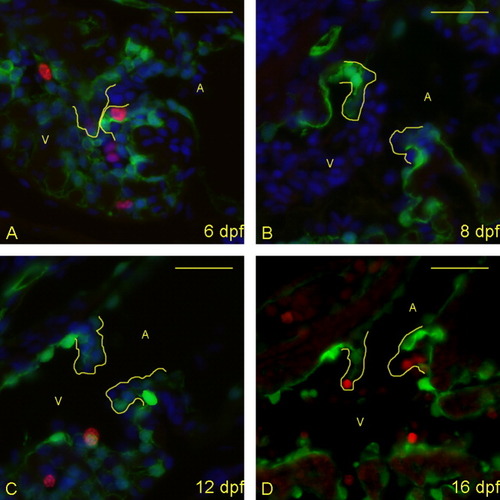
Proliferative cells are seen in the atrioventricular (AV) boundary at 6 and 16 days postfertilization (dpf), but not between these times. A-D: Five micrometer paraffin sections of flk1::GFP transgene-carrying zebrafish embryos/larvae were imaged to identify proliferating cells in the AV boundary and intra-luminal structure. Atria (A) and ventricles (V) are labeled (yellow letters), and yellow lines outline the extent of the AV structure (cushion and/or valve). Staining identifies cell nuclei (DAPI; blue), proliferative cells (anti-bromodeoxyuridine [BrdU]; red), and endothelial cells (anti-GFP; green). (DAPI not shown in panel D to emphasize BrdU-positive cells). Proliferative cells are seen in the developing valves at 6 dpf (A) and 16 dpf (D), but not 8 and 12 dpf (B,C). Scale bar = 20 μm. |
|
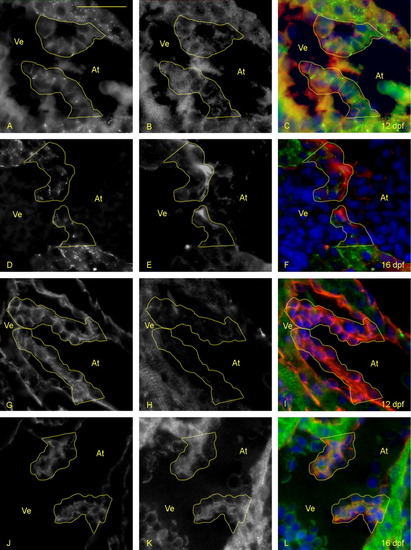
Valve leaflet cells become intermediate or mesenchymal between 12 and 16 days postfertilization (dpf). A-L: Five-micrometer paraffin sections of flk1::GFP (GFL, green fluorescent protein) transgene-carrying zebrafish embryos/larvae were immunostained to identify epithelial and mesenchymal cell phenotypes in the developing leaflets. Atria (At) and ventricles (Ve) are labeled (yellow letters), and yellow lines outline the extent of the atrioventricular (AV) valves. A-F: Staining identifies ZO-1 (A, D; green in C and F), focal adhesion kinase (B, E; red in C and F), and cell nuclei (DAPI: blue in C and F). The presence of ZO-1 reveals tight junctions that form to make cell to cell connections, which is indicative of an epithelial phenotype. FAK reveals whether there are transient (punctate) or long lived (large) cell:matrix adhesions occurring between these cells. A-C: At day 12, AV valve cells are organized, have ZO-1-containing tight junctions, and punctate FAK staining. D-F: By day 16, cells are less organized and some lack ZO-1-containing junctions. G-L: Staining identifies pancytokeratin (G, J; red in I and L), Vimentin (H, K; green in I and L), and cell nuclei (DAPI, 4′,6-diamidine-2-phenylidole-dihydrochloride; blue in I and L). G-I: At 12 dpf, the valves have a high level of pancytokeratin staining indicating that surrounding cells have a predominantly epithelial phenotype. J-L: By 16 dpf, a large increase in Vimentin is seen, indicating a shift toward mesenchymal cell phenotypes. Scale bar = 20 μm in A. |
|
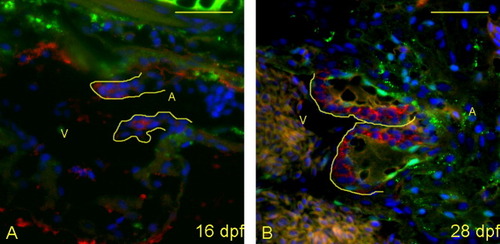
Maturation of the valve leaflets initiates between 16 and 28 days postfertilization (dpf). A,B: Five-micrometer paraffin sections of zebrafish larvae were imaged to identify the structural appearance of the AV boundary and intraluminal structure. Atria (A) and ventricles (V) are labeled (yellow letters), and yellow lines outline the extent of the atrioventricular (AV) structure (cushion and/or valve). Cells were stained with DAPI (4′,6-diamidine-2-phenylidole-dihydrochloride; blue), anti-collagen II (green) and anti-versican (red). A: At 16 dpf, the valve leaflets are highly cellular, with little collagen or versican present. B: By 28 dpf, the leaflets start to thicken with deposition of significant collagen fibers and versican. |
|
Larger larvae have more pronounced maturation of the valves at 28 days postfertilization (dpf). A-D: Five-micrometer paraffin sections of flk1:GFP (GFL, green fluorescent protein) transgene-carrying zebrafish larvae were imaged to identify the structural appearance of the atrioventricular (AV) boundary and intra-luminal structure. Atria (A) and ventricles (V) are labeled (yellow letters), and yellow lines outline the extent of the AV structure (cushion and/or valve). A,B: Sections were immunostained with DAPI (4′,6-diamidine-2-phenylidole-dihydrochloride; blue), anti-Versican (red) and anti-Collagen I (green). Whereas the smaller larva (A) has started deposition of Versican in the leaflet, the larger larva (B) has thickened considerably more, with increased deposition of Versican and Collagen I. C,D: From separate larvae than shown in A,B, sections were stained with DAPI (blue), anti-GFP (red), and anti-Collagen II (green). Again, the larger larva (D) has significantly more deposition of collagen than the smaller larva (C), despite being at the same age. Scale bar = 20 μm. |