- Title
-
Selenoprotein N is required for ryanodine receptor calcium release channel activity in human and zebrafish muscle
- Authors
- Jurynec, M.J., Xia, R., Mackrill, J.J., Gunther, D., Crawford, T., Flanigan, K.M., Abramson, J.J., Howard, M.T., and Grunwald, D.J.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
Loss of sepN function disrupts muscle differentiation in the zebrafish embryo. (A?E) sepN is expressed transiently in newly formed somites, adaxial cells (ad), presomitic mesoderm (PSM), and the notochord (ntc). sepN expression in (A and D) 10.5 hpf (two-somite stage), (B and E) 16 hpf, and (C) 24 hpf WT embryos. (A and B), Dorsal views with rostral up; (C) lateral view with rostral left. (D and E) Transverse sections through embryos at the levels indicated in B and C, respectively. (F and G) Three sequential frames illustrate that morphants, unlike WT embryos, rarely move >0.5 cm in response to touch. (H and I) Transmission electron micrographs of fast muscle fibers of 24 hpf (H) WT and (I) sepN morphant embryos indicate disruption of sarcomere organization in morphants. (J and K) Superficial slow muscle fibers of 24-hpf embryos stained with S58 antibody. (L?O) Development of sepN-depleted muscle fibers in WT hosts. sepN-depleted slow (L and M) or fast (N and O) dye-labeled muscle cells (red) were analyzed in mosaic embryos at 24 hpf stained with F59 or F310 antibody (green), respectively. (L and N) Dye-labeled donor cells; (M and O) merged views. *, normal-appearing fibers; arrows, dysmorphic donor fibers. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Slow muscle fiber formation is reduced in sepN morphants. (A?F) Somitic muscle in midtrunk region of 24-hpf WT and sepN morphant embryos. (A and B) S58+ slow muscle fibers and (C and D) 4D9+ slow muscle pioneer fiber nuclei are present in reduced numbers in sepN morphants. Staining for dystrophin expression (E and F) reveals ?u-shaped? somites in sepN morphant embryos. (G?J) Expression of myogenic lineage genes in adaxial cells of three-somite stage embryos. myoD (G and H) and α-cardiac actin (aca; I and J) are expressed discontinuously and at reduced levels in the adaxial cell population of sepN morphants. (A?F) Lateral views, rostral left. (G?J) Dorsal views, rostral left. EXPRESSION / LABELING:
PHENOTYPE:
|
|
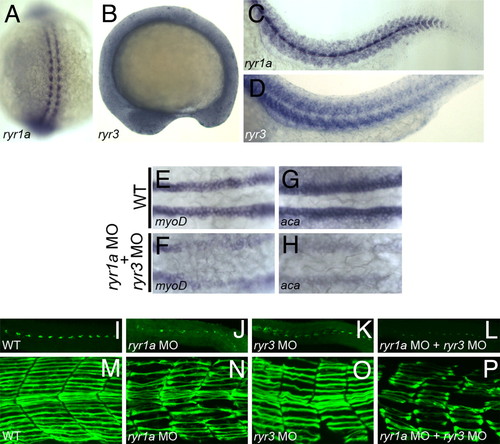
Ryanodine receptors and SepN have similar functions in muscle formation. (A?D) Expression of ryr1a and ryr3 transcripts in 15-somite (A and B) and 24 hpf (C and D) embryos. (E?H) Expression of the myogenic lineage genes, myoD and α-cardiac actin, is greatly reduced in the adaxial cell population of ryr1a; ryr3 double morphants. (I?L) 4D9+ slow muscle pioneer fiber nuclei and (M?P) F59+ slow muscle fibers are reduced in number and irregularly shaped in 24-hpf embryos depleted for RyR1a, RyR3, or both RyRs. (A) Dorsal view, rostral up. B?D and I?P, lateral views, rostral left. (E?H) Dorsal views, rostral left. EXPRESSION / LABELING:
PHENOTYPE:
|
|
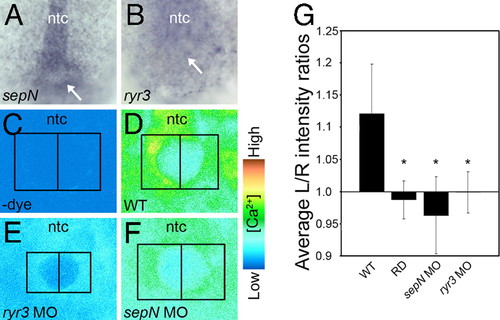
SepN and RyR3 are required for calcium signaling at the KV. (A and B) sepN and ryr3 transcripts are present in and around the KV (indicated by arrow) at the base of the notochord (ntc). (C?F) Visualization of free calcium in the region of the KV in 5- to 8-somite embryos. Images shown represent a single confocal section, taken from a dorsal view at the plane of the maximal apparent diameter of the KV. (C) Without calcium-sensitive dye, fluorescence is not detected. (D) Elevated levels of free calcium are detected on the left compared with the right side of the KV in WT embryos. (E) Loss of ryr3 function results in a dramatic overall reduction of free calcium. (F) Loss of sepN function results in complete loss of asymmetric distribution of free calcium. (G) For quantitative analyses, left- and right-side intensity values were determined for each embryo by integrating pixel values within symmetric boxed areas abutting the KV midline as indicated in C-F. Left/right intensity ratios were determined for each embryo [WT controls, n = 17; rhodamine dextran-injected (RD), n = 11; sepN MO-injected, n = 22; ryr3 MO-injected, n = 19] and average ratios are graphed. *, significant difference from WT ratio (P < 0.001 using paired Student′s t test). Error bars are ± SD. EXPRESSION / LABELING:
|
|
SepN genomic structure and morpholino inhibition of SepN expression. (a) Genomic structure of the zebrafish sepN locus containing conserved elements including a selenocysteine codon (Sec), a Sec Redefinition Element (SRE), and a selenocysteine insertion sequence (SECIS) element. * indicates stop codon; red bars indicate sbMOs; arrows indicate primers used to detect sepN transcripts by RT-PCR. (b) RT-PCR analysis of sepN transcripts in 24 hpf WT, MO1- or MO2-injected embryos. *,WT sepN amplicon; arrows, intron-included amplicons; m, markers. (c and d) sepN RNA levels are reduced in MO1-injected embryos. Lateral views with rostral left. EXPRESSION / LABELING:
|
|
Loss of SepN does not affect neural patterning or motor neuron projections. Expression of tal2 (a and b), isl2 (c and d), and ret (e and f) in 24 hpfWT and sepN morphants. Znp1 immunoreactivity in motor neurons in 24 hpf WT (g) and sepN (h) morphants. EXPRESSION / LABELING:
|
|
Loss of SepN does not affect cell death among adaxial cells. Cell death was measured in somitic mesoderm of three-somite stage WT (a) or sepN morphant (b) embryos. As a control to detect cell death that might occur whenever adaxial cell differentiation was blocked, embryos treated with 100 μM cyclopamine (c) were also examined. Adaxial cells form parallel rows of cells that lie immediately adjacent to the notochord and were visualized by a combination of staining for all nuclei (TO-PRO-3, blue) and for the No Tail protein, a marker of notochord (ntc) nuclei (α -Ntl, red). Apoptotic cells were visualized with TUNEL assay (green). Arrows indicate TUNEL-positive cells in the adaxial region, observed in similar numbers in control and treated embryos. Dorsal views with rostral left. |
|
Determination of transcript production in ryr morphant embryos. (a) Embryos were mock-injected (WT) or injected with a combination of two ryr1a sbMOs directed at the exon 2 splice acceptor and the exon 3 splice donor sites. RNA was analyzed from two independent injected groups, MOa and MOb. Transcripts were analyzed by RT-PCR at 24 hpf for production of mature erm or ryr1a RNA. Two pairs of exon-specific primers (E2-E3 and E3-E6) were used to detect different regions of the ryr1a transcripts. No WT ryr1a transcripts were detected in morphants. (b) Embryos were mock-injected (WT) or injected with a ryr3sbMOdirected at the exon 1 splice donor site. Transcripts were analyzed by RT-PCR at 24 hpf for production of mature erm or ryr3 RNA.Apair of exon-specific primers (E1-E2) was used to detect ryr3 transcripts. Decreased levels of WT ryr3 transcripts were detected in morphants. Minus sign indicates negative control reactions performed without cDNA synthesis. EXPRESSION / LABELING:
|
|
SepN and RyRs have more than additive effects on slow fiber formation. (a-c) Mild reduction of RyR1a or RyR3 levels with low doses of MO has no detectable effect on morphology of F59+ slow muscle fibers. (d-f) Mild reduction of RyR1a or RyR3 in embryos depleted of SepN leads to significant enhancement of the disrupted slow muscle fiber phenotype. Lateral views, rostral left. |
|
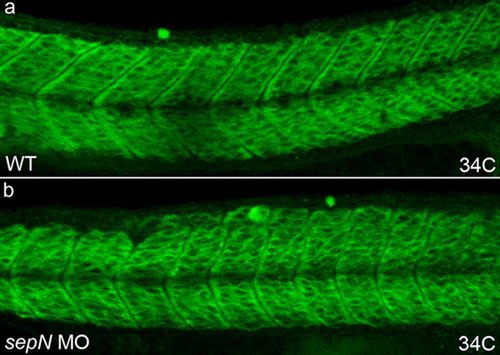
SepN-depleted andWTcontrol embryos have similar expression of RyR proteins.WT(a) and SepN MO-injected (b) embryos were analyzed for expression of RyR proteins by IHC with the 34C antibody. Lateral views, rostral left. |