- Title
-
APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development
- Authors
- Goessling, W., North, T.E., Lord, A.M., Ceol, C., Lee, S., Weidinger, G., Bourque, C., Strijbosch, R., Haramis, A.P., Puder, M., Clevers, H., Moon, R.T., and Zon, L.I.
- Source
- Full text @ Dev. Biol.
|
APC loss has differential effects on liver development. Zebrafish embryos were analyzed at 72 hpf. (A?C) Fluorescence microscopy of progeny of an APC+/-; lfabp:GFP incross revealed differences in liver size. (D) Graphic representation of the liver phenotypes (5 independent clutches; n = 584) shows a Mendelian distribution. (E?G) Total hepatocytes per embryo were quantified by flow cytometry for GFP in each APC genotype (green gate) and confirmed the differential effects of APC loss on liver cell number (APC+/+ 1.00 ± 0.37% (% GFP+ hepatocytes of 20,000 total embryo cells counted ± SD); APC+/- 2.65 ± 0.62%; APC-/- 0.20 ± 0.10%; ANOVA, n = 10, p < 0.00001); (H) APC+/- embryos have significantly more hepatocytes than wild-type controls, while APC-/- embryos have no GFP+ hepatocytes. (I-K) H+E liver sections (10 μm) from wild-type, APC+/-, and APC-/- embryos (40x) corroborated the effects of APC mutations; (L) APC+/- embryos had increased hepatocytes per section compared to controls (93.3 ± 20.4 vs. 39.7 ± 3.1; t-test, n = 5, p = 0.004). EXPRESSION / LABELING:
PHENOTYPE:
|
|
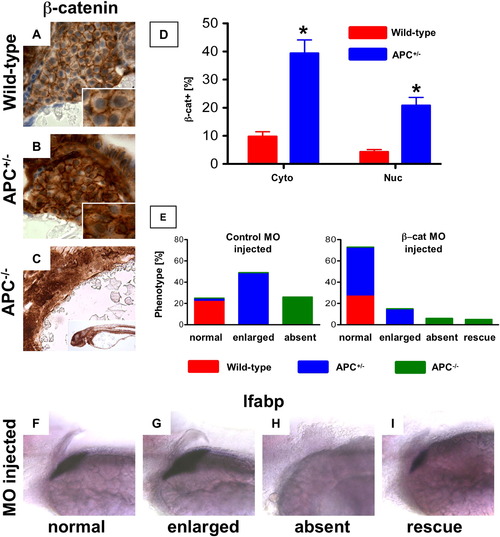
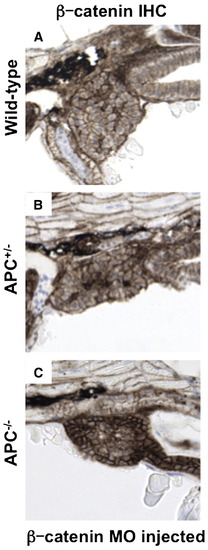
β-catenin levels mediate differential liver phenotypes in APC mutant zebrafish. (A?D) IHC for β-catenin at 72 hpf (40x, close-up as inset) revealed an increase in both cytoplasmic (APC+/-: 39.4 ± 13.4% (% of hepatocytes with cytoplasmic β-catenin ± SD) vs. 9.8 ± 3.8%; n = 5, p = 0.0006) and nuclear staining (20.8 ± 8.2% vs. 4.3 ± 1.8%; n = 5, p = 0.0012) in the hepatocytes of APC+/- embryos compared to wild-type. While liver-specific IHC could not be performed in the APC-/-embryos, β-catenin staining was widely positive in the region of the endoderm. (E?I) MO (40 μM) injected in the progeny of an APC+/- incross at the one-cell stage revealed a shift in liver phenotype distribution. (E) A graphical depiction of the shift in distribution of liver phenotypes with correlated genotypes in β-catenin MO injected embryos compared to controls. Distribution of control MO phenotypes (genotype): 59/234 normal (92% APC+/+, 8% APC+/-), 114/234 big (APC+/-), 61/234 absent (APC-/-); β-catenin MO phenotypes: 156/211 normal (40% APC+/+, 60% APC+/-), 32/211 big (APC+/-), 13/211 absent and 10/211 rescue (APC-/-). (F?I) Representative lfabp in situ hybridization phenotypes observed in β-catenin MO injected embryos at 72 hpf. EXPRESSION / LABELING:
PHENOTYPE:
|
|
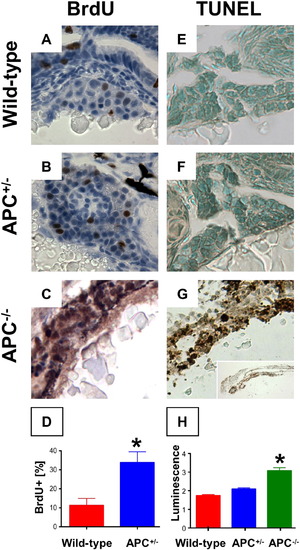
Endodermal proliferation and cell death are dependent on β-catenin levels. (A?D) BrdU incorporation in liver sections corresponding to Figs. 1I, J was significantly upregulated in APC+/- embryos (33.8 ± 12.6% vs. 11.3 ± 7.4%; n = 5, p = 0.016, 40′x), and also in the endodermal region of APC-/- embryos. (E?H) TUNEL staining demonstrated no apoptosis in wild-type and APC+/- embryos, while a high number of TUNEL+ cells were found in the endoderm of APC-/- embryos. (L) Caspase-3 and 7 activity was significantly increased in APC-/- mutants compared to wild-type and APC+/- (ANOVA, n = 10/genotype, p < 0.00001). Significant differences are indicated with an asterisk (∗). EXPRESSION / LABELING:
PHENOTYPE:
|
|
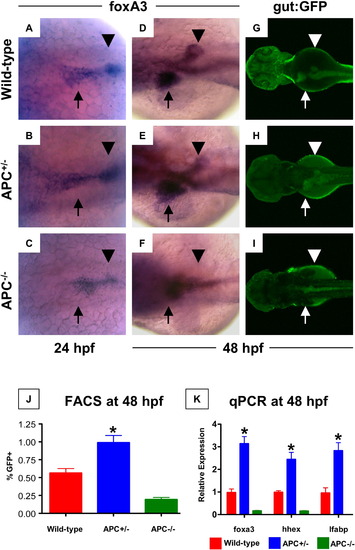
APC loss affects endodermal and hepatic progenitor cells. (A?C) In situ hybridization for foxa3 revealed changes in endodermal progenitor organization in APC mutant embryos as early as 24 hpf. (D?F) By 48 hpf, this led to a progressive increase in hepatic and corresponding decrease in pancreatic buds in the APC+/- embryos (35 altered/49 scored), while the APC-/- embryos failed to develop an organized endodermal pattern (23/25). (G?I) In vivo confocal fluorescence imaging of gut:GFP transgenic embryos at 48 hpf revealed similar effects on endodermal patterning. (J) FACS analysis demonstrated a doubling in the number of gut:GFP+ (green gate) endodermal progenitor cells in APC+/- embryos compared to wild-type controls; GFP+ cells were severely diminished in APC-/- embryos (APC+/+ 1.56 ± 0.063%; APC+/- 0.99 ± 0.099%; APC-/- 0.19 ± 0.089% of 20,000 cells analyzed; ANOVA, n = 10/genotype, p < 0.00001). (K) qPCR analysis confirmed the increased expression levels of foxa3, hhex and lfabp in APC+/- (blue) embryos and the depressed/absent expression in APC-/- (green) embryos at 48 hpf (ANOVA, n = 10/category, p < 0.05). |
|
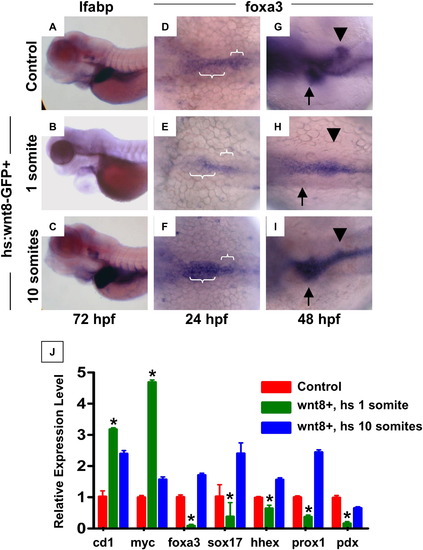
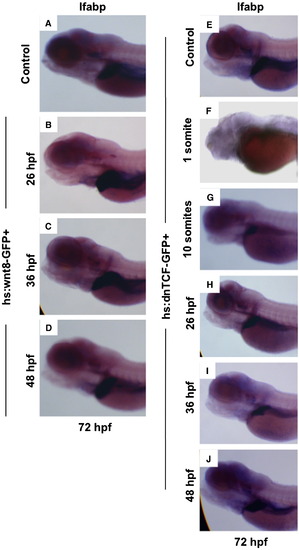
wnt signaling regulates endoderm development prior to liver specification. Heat-shock inducible wnt8-GFP or control embryos were incubated for 20 mins at 38 °C at various time points between 50% epiboly and 48 hpf, and were analyzed for alterations in liver size at 72 hpf by in situ hybridization for lfabp. (A, B) Induction of wnt8 at 1 somite (10 hpf) led to grossly abnormal embryos, with cardiac edema and absent livers (49 altered/ -52 scored). (C) Induction of wnt8 at 10 somites (14 hpf) resulted in a markedly enlarged livers compared to controls (141/173). (D?F) foxa3 expression at 24 hpf following heat-shock at 1 somite (10 hpf) revealed a failure of cells to converge at the midline and expand in the a?p direction (36/45); heat-shock at 10 somites (14 hpf) led to enhanced foxa3 expression (52/67). (G?I) Expression of foxa3 at 48 hpf revealed a failure to form organized liver and pancreatic buds after wnt8 induction at 1 somite (10 hpf) (45/61); wnt8 induction at 10 somites (14 hpf) led to an increased liver and decreased pancreatic anlage (52/78). (J) qPCR analysis of the expression levels of wnt target (cyclind1, cmyc), endodermal (foxa3, sox17), hepatic (hhex, prox1) and pancreatic (pdx1) progenitor genes at 48 hpf in controls (red) and following wnt8 induction at either 1 somite (10 hpf; green) or 10 somites (14 hpf; blue). All changes were statistically significant compared to controls (ANOVA, n = 10, p < 0.05). EXPRESSION / LABELING:
|
|
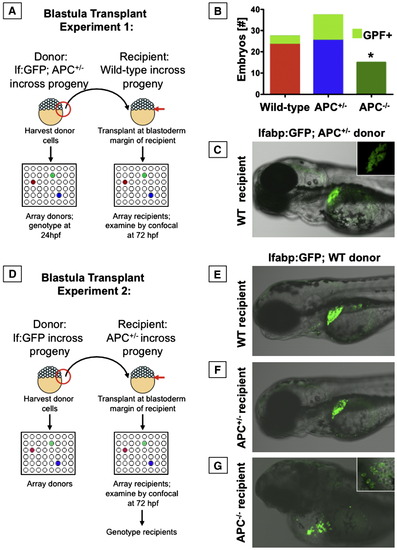
APC has cell autonomous effects on endoderm development. Blastula transplant experiments; embryos were analyzed at 72 hpf. (A) Schematic depiction of blastula transplant experiments with each APC genotype as donor. (B) Graphic summary of the number of recipient embryos that received each donor genotype; the fraction of embryos that showed donor contribution to the liver is highlighted in light green. No APC-/- donor cells contributed to liver formation (Fisher's exact, p = 0.025). (C) Mosaic livers showed green hepatocytes interspersed with unlabeled cells. (D) Schematic depiction of blastula transplant experiments with different APC genotypes as recipients. (E, F) lfabp:GFP donor cells transplanted into both wild-type or APC+/- hosts gave rise to mosaic livers. (G) In an APC-/- host, lfabp:GFP hepatocytes developed, but could not rescue liver development. The inset shows formation of chains of hepatocytes near the heart and around the yolk sac. Significant differences are indicated with an asterisk (∗). |
|
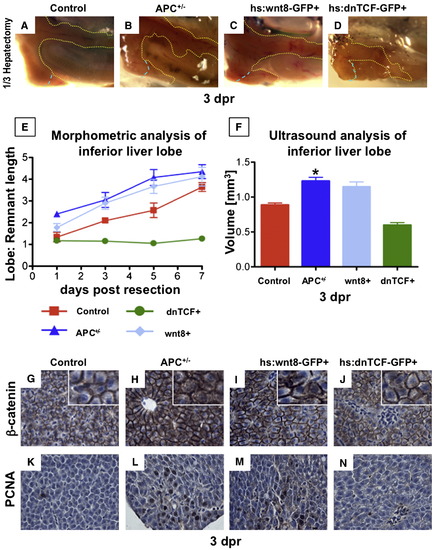
Liver regeneration following partial hepatectomy in adult zebrafish is enhanced by wnt activation. (A?D) En-bloc dissection of APC+/- and hs:wnt8 (induced from 6 to 18 hpr) fish at 3 dpr revealed enhanced regeneration, while inhibition of signaling by dnTCF (induced from 6 to 18 hpr) resulted in severely impaired regenerative capacity. (E) Quantitative analysis of the liver lobe:remnant ratios in zebrafish at days 1, 3, 5 and 7 post resection reveals enhanced regenerative kinetics caused by wnt/β-catenin activation in APC+/- (blue triangle) and wnt8 (light blue diamond) fish, while dnTCF (green circle) inhibits liver regeneration. (F) In vivo volume measurements of the inferior liver lobe by high-frequency ultrasound at 3 dpr confirmed the regenerative advantage in APC+/- (blue) and wnt8 (light blue) fish and the decreased regenerative capacity in dnTCF fish (green) (ANOVA, n = 3/genotype, p < 0.05). (G?J) IHC in these fish at 3 dpr revealed increased cytoplasmic and nuclear β-catenin staining in APC+/- and hs:wnt8 fish. (K?N) Hepatocyte proliferation measured by PCNA staining was increased in APC+/- and wnt8 fish, but absent in dnTCF fish. Significant differences are indicated with an asterisk (∗). |
|
Mutants developed a small liver with moderate β-catenin levels. EXPRESSION / LABELING:
PHENOTYPE:
|
|
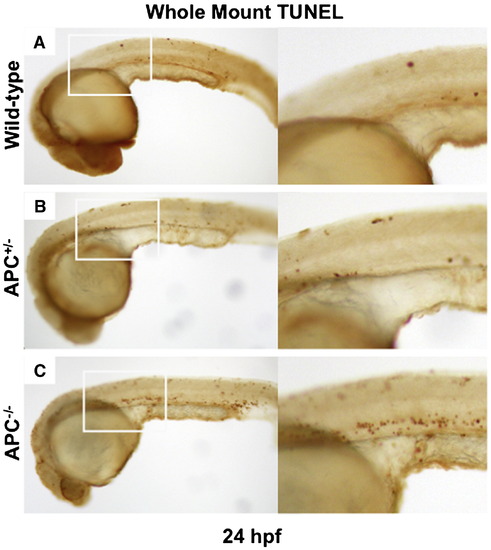
Apoptotic cell death does not occur during early endoderm development in APC-/- mutants. (A-C) Whole mount TUNEL staining at 24 hpf revealed few apoptotic cells in wild-type and APC+/-- embryos. APC-/-- embryos demonstrate increased numbers of TUNEL positive cells, without massive apoptotic cell death in the endoderm at this stage. |
|
APC loss affects hepatic progenitor cells. (A?C) In situ hybridization for hhex at 26 hpf demonstrated early effects of APC loss on hepatic progenitor cells. |
|
Time-dependent modulation of wnt activity affects and liver formation and liver and pancreatic progenitors. (A?J) In situ hybridization for lfabp at 72 hpf. (A?D) wnt8 induction at time points from 26 to 48 hpf increased liver size. (E,F) Activation of dnTCF at the 1 somite (10 hpf) stage led to severe morphological defects (46/51). (G?J) Induction of dnTCF impaired liver growth at all stages examined, with the most significant effects occurring at 10 somites (14 hpf; 122/141). |
|
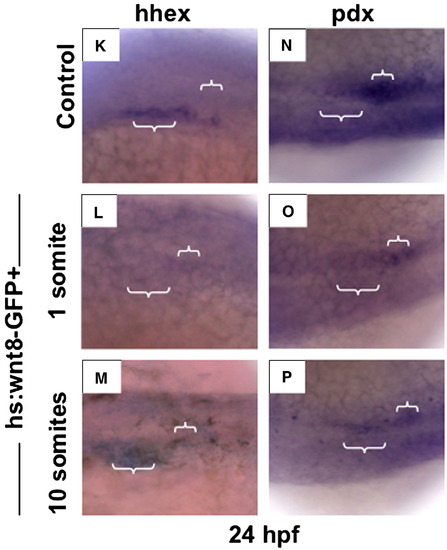
(K?M) Induction of wnt8 at 1 somite (10 hpf) resulted in inhibition of hhex expression at 24 hpf (39/51), while heat-shock at 10 somites (14 hpf) caused significant expansion of hhex (60/72). (N?P) Expression of pdx1 was diminished following wnt8 induction at both 1 (29/37) and 10 somites (14 hpf; 42/54). EXPRESSION / LABELING:
|
|
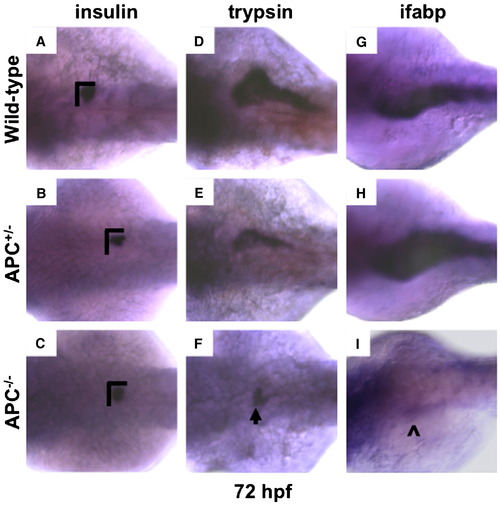
APC loss affects endodermal fate. (A?C) Insulin and (D?F) trypsin in situ hybridization at 72 hpf in all APC genotypes revealed a decrease in endocrine and exocrine pancreas size in both APC+/- (insulin: 51/65; trypsin: 68/74) and APC-/- (insulin: 25/29; trypsin: 32/32) mutants. The dimensions of the endocrine pancreas in wild-type embryos are indicated with black bars for comparison. An arrowhead highlights a small area of trypsin expression in one homozygous mutant embryo. (G-I) ifabp expression is affected in a similar manner to that seen with lfabp; APC+/- embryos had an increase in ifabp (31/55), while there was an absence of expression in homozygous mutants (28/29). The (^) indicates the presence of minimal ifabp expression found in a single embryo. EXPRESSION / LABELING:
PHENOTYPE:
|
|
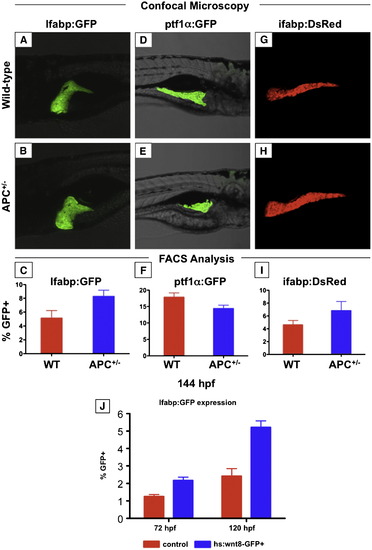
The effects of wnt activation on mature endodermal organs persist into the larval stage. (A?I) Confocal microscopy and FACS analysis of live zebrafish larvae at 144 hpf (6 dpf). (A?C) APC+/-; lfabp:GFP larvae maintain increased liver size; this is confirmed by FACS analysis. (D?F) The pancreas of APC+/-; ptf1α:GFP larvae remains smaller than that of wild-type siblings. (G?H) The effect on the intestine as indicated in ifabp:GFP reporter fish parallels that of the liver. (J) Induction of wnt8 after liver differentiation at 48 hpf increased the number of LFABP:GFP+ hepatocytes at 72 (wild-type 1.25 ± 0.12% vs. wnt8 2.42 ± 0.43%, n = 10, p < 0.0005) and 120 hpf (wild-type 2.17 ± 0.19% vs. wnt8 5.21 ± 0.37%, n = 10, p < 0.0005). EXPRESSION / LABELING:
|
|
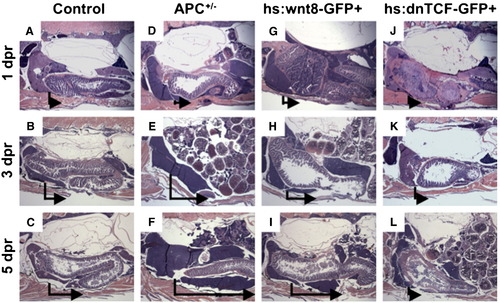
Wnt activation enhances liver regeneration in zebrafish. (A?L) Sagittal, H+E stained sections through adult zebrafish at 1, 3, and 5 dpr. APC+/- and hs:wnt8 fish demonstrate enhanced liver regeneration, while hs:dnTCF fish fail to regenerate liver. The vertical line in each panel indicates the resection margin. The horizontal arrow represents the extent of hepatic regrowth in the inferior lobe. PHENOTYPE:
|
Reprinted from Developmental Biology, 320(1), Goessling, W., North, T.E., Lord, A.M., Ceol, C., Lee, S., Weidinger, G., Bourque, C., Strijbosch, R., Haramis, A.P., Puder, M., Clevers, H., Moon, R.T., and Zon, L.I., APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development, 161-174, Copyright (2008) with permission from Elsevier. Full text @ Dev. Biol.