- Title
-
Complex Regulation of cyp26a1 Creates a Robust Retinoic Acid Gradient in the Zebrafish Embryo
- Authors
- White, R.J., Nie, Q., Lander, A.D., and Schilling, T.F.
- Source
- Full text @ PLoS Biol.
|
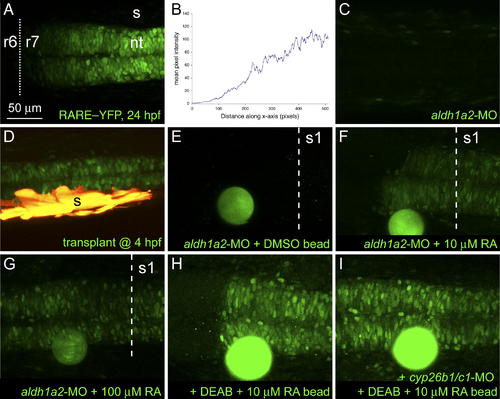
Responses of Embryos to Sources of RA. Long-range induction of a rare:yfp reporter. Confocal images of live embryos, dorsal view, anterior to the left. (A) rare:yfp expression in posterior hindbrain and spinal cord at 24 hpf with an anterior boundary at r6/7. (B) Quantification of fluorescence intensities in (A). (C) Lack of YFP expression in an aldh1a2 morphant embryo. (D) Rescue of YFP expression in a morphant by somitic mesoderm (yellow) transplanted during gastrulation. (E?I) Bead implantation. RA-coated beads were implanted anterior to the first somite at 18?19 hpf and imaged at 23?24 hpf. A DMSO-coated bead (E) failed to rescue YFP expression, whereas beads soaked in either 10 μM (F) or 100 μM (G) RA induced YFP at distances of up to 200 μm. (H and I) Inhibition of both cyp26b1 and cyp26c1 in DEAB-treated embryos leads to a symmetrical response from the rare:yfp reporter to a bead soaked in 10 μM RA (compare [H] with [I]). The dotted line indicates the r6/7 boundary, the dashed line, the anterior border of somite 1. nt, neural tube; s, somites. EXPRESSION / LABELING:
|
|
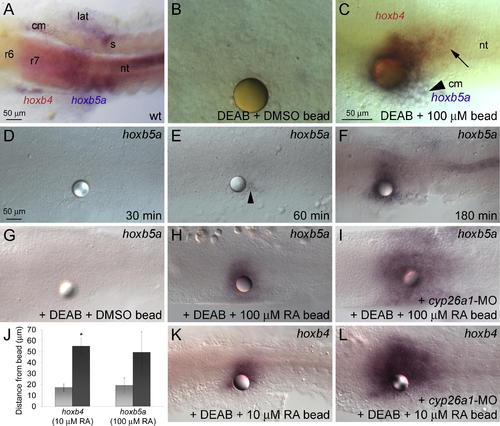
Concentration-Dependent Induction of hox Gene Expression by RA (A) In wild-type (wt) embryos, hoxb4 expression in the neural tube (nt) extends to the r6/7 boundary, whereas the limit of hoxb5a expression is level with the first somite (s). hoxb5a is also expressed in axial, lateral (lat), and cranial (cm) mesoderm. (B and C) Beads soaked in 100 μM RA were implanted at 1?3 somites (10.3?11 hpf) into DEAB-treated embryos, fixed 3 h later, and assayed for hox expression. Dorsal views, showing in situ hybridization with probes for hoxb4 and hoxb5a at 14 hpf. (B) DMSO-coated beads do not induce hox expression. (C) RA-coated beads induce hoxb5a in lateral mesoderm (arrowhead), hoxb5a/hoxb4 double-positive cells near the bead, and hoxb4 single-positive cells further away (black arrow). (D?F) Time-course of hoxb5a induction in response to a 100 μM RA bead: no induction 30 min post-implantation (D), a few cells near the bead after 60 min (arrowhead) (E), and strong expression further away after 180 min (F). (G?L) RA-induced degradation limits the range of RA signaling. RA beads were implanted at 1?3 somites (10.3?11 hpf) into DEAB-treated embryos, fixed 3 h later, and assayed for hox expression. Dorsal views, showing in situ hybridization with probes for hoxb5a (G?I) or hoxb4 (K and L) at 14 hpf. (G) Implantation of a DMSO bead has no effect on gene expression in DEAB?treated embryos. (H and I) A 100 μM RA bead placed into a DEAB-treated, cyp26a1 morphant (I) induces hoxb5a over a much larger area than a DEAB-treated control (H). (J) Graph showing an increase in the range of hox induction in cyp26a1 morphant, DEAB-treated embryos (black bar) as compared to DEAB-treated (light grey bar). The asterisk (*) indicates significantly different from DEAB-treated controls (p < 0.05), using the Student t-test. (K and L) A 10 μM RA bead placed into a cyp26a1 morphant induces hoxb4 over a much larger area. |
|
Regulation of Cyp26s by RA (A) Loss of cyp26a1 expression in cranial mesoderm in DEAB-treated embryos and lack of induction by a DMSO-soaked bead. (B) Induction of cyp26a1 expression by an RA bead; inset, optical section showing cyp26a1 induction in both the neural tube (nt), lying above the notochord (n), and lateral mesoderm (white arrowhead). The asterisk (*) indicates the bead. (C) Weak induction of cyp26b1 (black arrowhead). (D) No induction of cyp26c1. |
|
RA-Dependent Expression of cyp26a1 Wild-type (wt) and DEAB-treated embryos were stained for extended periods of time for cyp26a1 expression to investigate low-level expression. Top, dorsal view; bottom, lateral view. (A and C) Weak expression posterior to the presumptive midbrain (arrow to arrowhead). (B and D) This expression is lost in DEAB-treated embryos. EXPRESSION / LABELING:
|
|
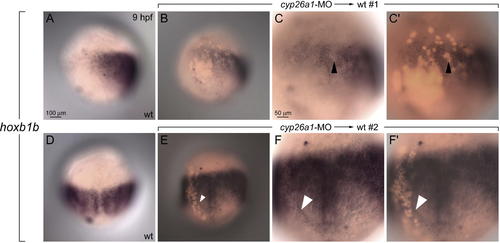
cyp26a1 Is Required Outside of the Anterior Neural Domain (A and D) hoxb1b expression in wild-type (wt) embryos. (A?C′) Lateral view, anterior to top, dorsal to right. (D?F′) Dorsal view, anterior to top. (B, C′, E, and F′) Merged brightfield and fluorescent images show positions of transplanted cyp26a1-deficient cells labeled with lysine-fixable rhodamine dextran (red). (B?C′) Transplants of cyp26a1-deficient cells located within the endogenous hoxb1b domain cause a nearly cell-autonomous decrease in hoxb1b expression (black arrowheads). (E?F′) Transplants of cyp26a1-deficient cells also cause changes in hoxb1b expression in adjacent host cells (white arrowheads). EXPRESSION / LABELING:
|
|
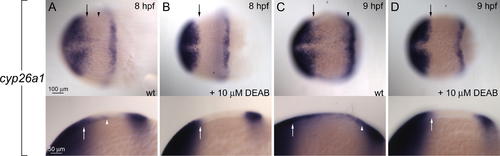
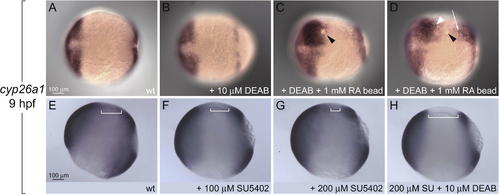
Regulation of cyp26a1 by RA and Fgfs (A and B) Anterior and marginal expression domains of cyp26a1 expression are unchanged by 10 μM DEAB. Anterior is to the left in all panels. (C and D) RA beads (black arrowheads) induce cyp26a1 expression in DEAB-treated embryos far from (white arrowhead and arrow in [D]), but not adjacent to the bead. (E?H) Fgf signaling sets the posterior boundary of cyp26a1 expression. (E) cyp26a1 expression in wild type (wt) (lateral view, anterior to the left). (F and G) Expression in SU5402-treated embryos shifts posteriorly in a concentration-dependent manner (compare brackets in [E], [F] and [G]). (H) The posterior shift caused by blocking Fgf signaling is abolished by DEAB treatment. EXPRESSION / LABELING:
|
|
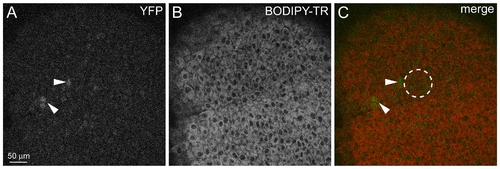
Induction of rare:yfp Reporter at Gastrula Stage (A?C) Implantation of a bead soaked in 10 mM RA at 8 hpf induces YFP expression after 2 h. Single confocal sections show nuclear YFP expression (A) and cells labeled with BODIPY-TR (B). (C) Merged image. dashed circle indicates the position of the implanted bead. |
|
Response of cyp26a1 to Global RA Treatment (A?D) cyp26a1 is up-regulated in a concentration-dependent manner that also depends on a cell's position in the embryo (compare [B], [C], and [D] to [A]). EXPRESSION / LABELING:
|
|
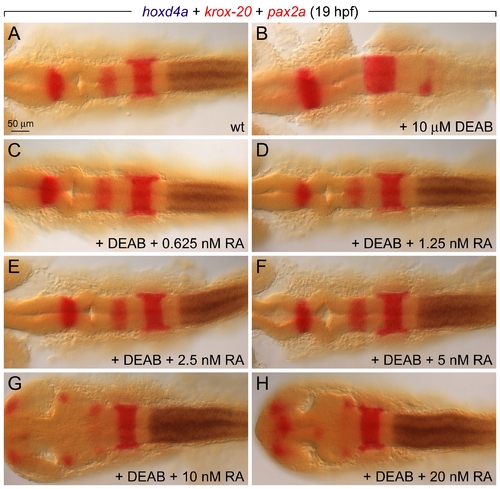
Global RA Treatments Rescue Hindbrain Segmentation over a Wide Range of Concentrations. Embryos were treated at 4 hpf with DEAB either with or without a low concentration of RA between 0.625?20 nM, fixed at 19 hpf, and pattern was assessed by in situ hybridization for hoxd4a (blue; r6/7 boundary), krox-20 (egr2b, red; r3 and r5), and pax2a (red; midbrain?hindbrain boundary). (A) Wild-type expression. (B) DEAB-treated embryos show loss of r5?r7. (C?F) Concentrations from 0.625?5 nM rescue wild-type patterning to the same extent. (G?H) Higher concentrations produce anterior defects. EXPRESSION / LABELING:
|