- Title
-
Retinoic acid activates myogenesis in vivo through Fgf8 signalling
- Authors
- Hamade, A., Deries, M., Begemann, G., Bally-Cuif, L., Genet, C., Sabatier, F., Bonnieu, A., and Cousin, X.
- Source
- Full text @ Dev. Biol.
|
Repression and activation, respectively, of the expression of myoD by DEAB and RA. Flat-mounted embryos, anterior is to the left. (A?B) Comparison of myoD expression in wild-type (A) and neckless (nls; B) siblings at the 12-somite stage. At this stage, myoD is expressed in the lateral aspect of somites (arrowhead) and two rows of adaxial cells (arrows) in wild-type embryos (A) and krox20 is expressed in rhombomeres 3 and 5 (asterisks). The shorter distance between rhombomere 5 and trunk mesoderm (myoD) has been used to identify nls embryos (brackets in panels A and B). Expression of myoD is reduced in nls embryos (B). (C) Closer view restricted to the trunk showing expression of myoD in 10-somite stage embryo. (D) In DEAB-treated embryos, myoD expression is reduced in somites, as well as in adaxial cells (white arrowhead). (E) In RA-treated embryos, myoD expression is up-regulated in somites and strongly induced in anterior psm (bracket). (F) Control at the 12-somite stage. (G?H) Incubation in retinal (Ral) produces the same phenotype as incubation in RA. Embryos incubated in DEAB and Ral are undistinguishable from control embryos demonstrating that Ral needs to be converted locally in RA by Raldhs to induce myoD expression. psm, presomitic mesoderm. Scale bars: in panel A, 100 μm for panels A?B; in panel C, 100 μm for panels C?H. EXPRESSION / LABELING:
|
|
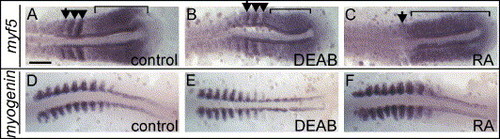
Regulation of early and late differentiation markers by RA. 10-somite stage embryos are displayed as in Fig. 2. (A) myf5 is expressed in wild-type posterior psm (bracket) and is re-expressed in forming somites (arrowheads). Somitic expression decreases as somites mature (arrow indicates the posterior border of last formed somite). (B) In DEAB-treated embryos, myf5 is reduced in both psm (bracket) and somites (arrowheads). (C) In RA-treated embryos, myf5 expression encompasses the entire psm (bracket; arrow indicates the posterior border of last formed somite) and is absent in somites. (D) myogenin expression in wild-type embryos resembles that of myoD, it is decreased in posterior somites in DEAB-treated embryo (E) and increased in somites of RA-treated embryo (F). Scale bar: in panel A, 100 μm for panels A?F. EXPRESSION / LABELING:
|
|
Somite maturation but not segmentation pattern is regulated by RA. 15-somite (A?C) and 10-somite (D?L) stage embryos are displayed as in Fig. 2. (A?C) At the 15-somite stage, twist is expressed in sclerotome. twist expression is reduced in DEAB-treated embryos (B) and increased in RA-treated embryos (C). (D?I) The segmented pattern (arrows) of mesp-b (D?F) and papc (G?I) is not modified by either DEAB or RA treatments. (J?L) paraxis is expressed in anterior psm (bracket) and somites. Expression of paraxis in DEAB-treated embryos is similar to that in control (K) while it is extended posteriorly in RA-treated embryos (L; bracket). Scale bars: in panel A, 100 μm for panels A?C; in panel D, 100 μm for panels D?L. EXPRESSION / LABELING:
|
|
Differential response of slow- and fast-muscle differentiation depending on developing stage. 10- and 15-somite stage embryos were stained for myosin expression. At stages analysed, F59 antibody is specific for slow-myosin, F310 is specific for fast-myosin. (A?C) Dorsal views, anterior to the top, (D?I) lateral views, anterior to the top. (A) At 10-somite stage, slow-myosin is detected in two rows of adaxial cells (arrows). (B?C) Slow-myosin expression is decreased in DEAB-treated embryos (B), and similar to wild-type in RA-treated embryos (C). (D) In 10-somite stage, fast-muscle differentiation begins in anterior somites (dots indicate somites counted as positive in panel K). (E) Fast myosin expression is unchanged in DEAB-treated embryos. (F) Fast-myosin expression is increased in RA-treated embryos as well as the number of F310-positive somites (dots). (G?I) The same observation can be made at 15-somite stage for all three conditions. (J) Western blot analysis of fast myosin expression with antibody F310 revealed the presence of two bands corresponding to Myosin light chain (MLC), MLC3f (3f; 15 kDa) and MLC1f (1f; 22 kDa). At 10-somite stage, MLC1f is barely visible in control lane (lane C) while it is strongly expressed in RA-treated (RA lane). MLC3f is decreased in DEAB lane and increased in RA lane. At 15-somite, MLC1f can now be seen in DEAB lane albeit weaker than in control lane, while it is increased in RA lane compared to control. α-Tubulin (αT) has been used to compare loading in each lane. (K?L) Comparison of F310-positive somites (grey histogram) according to the number of somites (white histogram) at 10-somite (K) and 15-somite stage (L). DEAB treatment produces a significant reduction of F310-positive somites at the 15-somite stage while RA produces a significant increase at both 10- and 15-somite stages compared to control. The standard error is indicated. * Indicates number significantly different from wild-type (PKW < 0.001). Scale bar: in panel A, 100 μm for panels A?I. |
|
Induction of myoD by RA requires translation of a protein intermediate. 10-somite stage embryos, oriented as in Fig. 2. (A?B) RA activates expression of myoD in somites and psm (bracket) (B) compared to wild-type (A). (C?D) Cycloheximide (CHX) decreases expression of myoD in somites (C) and severely reduces myoD induction by RA in anterior psm (D). Scale bar: in panel A, 100 μm for panels A?D. EXPRESSION / LABELING:
|
|
Expression of myoD can be induced by RA in absence of Hh signalling. (A?C) 10-somite stage embryos displayed as in Fig. 2. At this stage, shh is expressed in embryo midline and no difference is observed upon DEAB or RA treatment. (D?G) Dorsal views of myoD expression in 10-somite stage embryos. (D, E) myoD fails to be expressed in adaxial cells in smu embryos (arrows in panel E). (F, G) In RA-treated embryos, myoD is induced in psm (bracket) in wild-types (F) and smu (G). Note that myoD remains absent from adaxial cells in RA-treated smu embryos (arrows). Scale bars: in panel A, 100 μm for panels A?C; in panel D, 100 μm for panels D?G. EXPRESSION / LABELING:
|
|
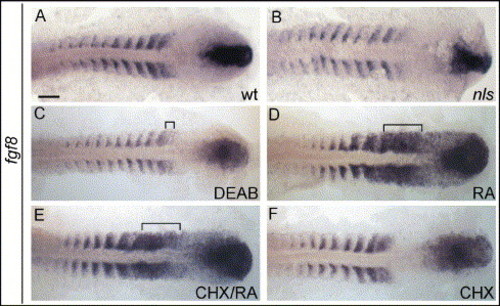
Repression and activation, respectively, of fgf8 expression by DEAB and RA. 10-somite stage embryos, oriented as in Fig. 2. (A) fgf8 is expressed in tailbud, anterior psm and anterior aspect of somites; expression in somites decreases as somites maturate. (B) In nls embryos, fgf8 expression is overall reduced. (C) In DEAB-treated embryos, fgf8 is strongly reduced in anterior psm (bracket) and somites. (D) In RA-treated embryos, fgf8 expression is increased in anterior psm (bracket). (E) Blocking of translation by prior incubation in cycloheximide (CHX/RA) does not prevent fgf8 activation by RA in psm (bracket). Scale bar: in panel A, 100 μm for panels A?F. EXPRESSION / LABELING:
|
|
Expression of genes involved in Fgf-signalling is regulated by RA. 10-somite stage embryos displayed as in Fig. 2. (A?C) In wild-type embryos, fgf17b is expressed in somites, anterior psm (bracket; black arrow indicates position of posterior border of last formed somite), tailbud (arrowhead) and adaxial cells (white arrow; A). In DEAB-treated embryos, fgf17b is reduced in psm, somites and adaxial cells (B); in RA-treated embryos, expression extends to a larger part of anterior psm (bracket) and is increased adaxial cells (white arrow; C). (D?F) In wild-type embryos, erm1 is expressed in tailbud, posterior psm and in somites, but is absent from anterior psm (bracket in panel D); in DEAB-treated embryos, erm1 expression is reduced in somites and posterior psm (E); in RA-treated embryos, erm1 is induced in anterior psm (F). Scale bar: in panel A, 100 μm for panels A?F. EXPRESSION / LABELING:
|
|
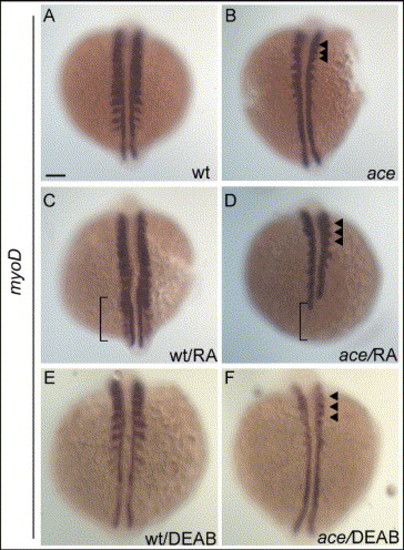
Induction of myoD by RA requires a functional Fgf8. 10-somite stage embryos are displayed as in Fig. 7. (A?B) Untreated embryos. In ace, expression of myoD is reduced in the lateral aspect of somites, but remains expressed in a domain close to adaxial cells in anterior somites (arrowheads in panel B). (C?D) RA-treated embryos; expression of myoD is strongly induced in somites and psm of wild-type embryos (bracket in panel C). In ace, expression of myoD is weak in somites (arrowheads) and reduced in adaxial cells at the level of psm (bracket; D). (E?F) DEAB-treated embryos; expression of myoD is reduced in somites of wild-type embryos (E) compared to untreated wild-type (A). Reduction is stronger in somites of ace embryos (F; arrowheads) compared to DEAB-wild-type (E) and untreated-ace embryos (B). Scale bar: in panel A, 100 μm for panels A?F. EXPRESSION / LABELING:
|
|
Fgf8 controls expression of myoD in the lateral part of somites. Dorsal views of embryos, anterior to the top (A?D, I?L, S?T); lateral views (E?H, M?R). (A?D) Comparison of myoD expression in wt and ace embryos at 6- and 10-somite stages. Absence of pax2.1 expression in the otic placodes identifies ace embryos (white arrowheads), other areas of pax2.1 expression can be seen in intermediate mesoderm (unchanged in ace). (A, B) At the 6-somite stage, myoD expression in lateral parts of somites of wild-type embryos (brackets; A) is reduced in ace embryos as well as expression in adaxial cells (arrowhead; B). (C, D) At the 10-somite stage, myoD is strongly expressed in lateral parts of somites (C) and is reduced in ace embryos (D). (E?H) Induction timing of fgf8 by RA. At 3-somite stage, fgf8 is expressed in the tailbud (white arrow) in wild-types (E). In RA-treated embryos, fgf8 expression is induced in anterior psm (black arrow) and increased in tailbud (white arrow; F). (G, H) At the 6-somite stage, expression appears in anterior psm (black arrow) and somites (arrowhead) (G). Following RA treatment, expression in anterior psm (black arrow) and somites (arrowheads, H) is increased. (I?L) Timing of myoD-induction by RA. (I, J) At the 3-somite stage, myoD expression in adaxial cells is not modified by RA. (K, L) At the 6-somite stage, myoD expression expands in lateral part of somites. At this stage, myoD expression is not modified by RA. (M?P) raldh2 is expressed in somites from 1- to 10-somite stages. (Q?R) In wild-type and ace embryos, cyp26a1 is expressed in tailbud to a similar extent (black arrow). Reduction of pax2.1 expression in mhb was used to identify ace mutant (white arrowheads). (S?T) raldh2 expression at the 10-somite stage is reduced in somites of ace embryos (T) compared to wild-type (S). Scale bars: in panel A, 100 μm for panels A?D; in panel E, 100 μm for panels E?L; in panel M, 100 μm for panels M?P; in panel Q, 100 μm for panels Q?R; in panel S, 100 μm for panels S?T. EXPRESSION / LABELING:
|
Reprinted from Developmental Biology, 289(1), Hamade, A., Deries, M., Begemann, G., Bally-Cuif, L., Genet, C., Sabatier, F., Bonnieu, A., and Cousin, X., Retinoic acid activates myogenesis in vivo through Fgf8 signalling, 127-140, Copyright (2006) with permission from Elsevier. Full text @ Dev. Biol.