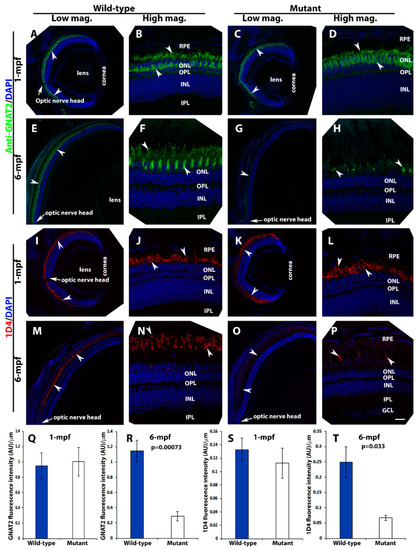
Loss of photoreceptors in pomt2 mutant retinas at 6-mpf but not 1-mpf. Zebrafish retinal sections at 1-mpf and 6-mpf were immunostained with GNAT2 antibody (green fluorescence, A–H) and 1D4 (red fluorescence, I–P). The sections were counter-stained with DAPI. To quantify loss of cone photoreceptors, GNAT2 and 1D4 immunoreactive intensities were measured (Q–T). (A,B) Wild-type GNAT2 immunostaining at 1-mpf. (C,D) Homozygous pomt2sny5+13 mutant GNAT2 immunostaining at 1-mpf showing similar immunoreactivity compared to the wild-type. (E,F) Wild-type GNAT2 immunostaining at 6-mpf. (G,H) Homozygous pomt2sny5+13 mutant GNAT2 immunostaining at 6-mpf showing reduced immunoreactivity compared to the wild-type. (I,J) Wild-type 1D4 immunostaining at 1-mpf. (K,L) Homozygous pomt2sny5+13 mutant 1D4 immunostaining at 1-mpf showing similar immunoreactivity compared to the wild-type. (M,N) Wild-type 1D4 immunostaining at 6-mpf. (O,P) Homozygous pomt2sny5+13 mutant 1D4 immunostaining at 6-mpf showing reduced immunoreactivity compared to the wild-type. (Q) GNAT2 immunofluorescence intensity (artificial units, AU) quantification of wild-type and mutant fish at 1-mpf (n = 3). There was no significant difference between wild-type and mutant retinas. (R) GNAT2 immunofluorescence intensity quantification of wild-type and mutant fish at 6-mpf (n = 3). GNAT2 immunoreactivity was significantly reduced in mutant retinas. (S) 1D4 immunofluorescence intensity quantification of wild-type and mutant fish at 1-mpf (n = 3). There was no significant difference between wild-type and mutant retinas. (T) 1D4 immunofluorescence intensity in the mutant retina was reduced at 6-mpf (n = 3). Arrowheads indicate examples of GNAT2 immunoreactivity (A-H) and 1D4 immunoreactiity (I-P). Arrows indicate location of optic nerve heads. Scale bar in P: 100 µm for all low magnification panels and 18.7 µm for all high magnification panels.
|

