Fig. 4
- ID
- ZDB-FIG-181012-29
- Publication
- Kustermann et al., 2018 - Loss of the novel Vcp (valosin containing protein) interactor Washc4 interferes with autophagy-mediated proteostasis in striated muscle and leads to myopathy in vivo
- Other Figures
- All Figure Page
- Back to All Figure Page
|
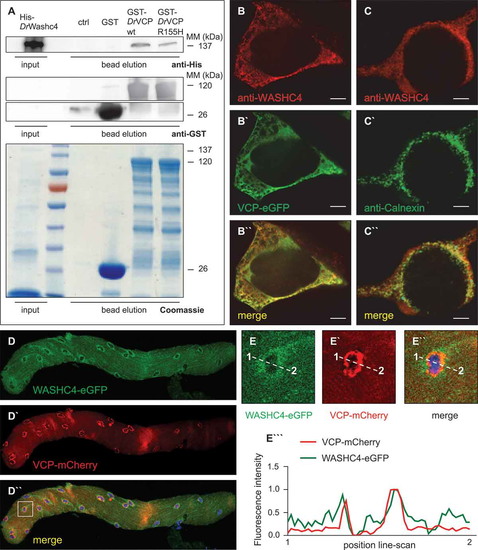
VCP and its interactor WASHC4 colocalize in HEK293T cells and mouse FDB fibers. (a) Western blot analysis of a representative Washc4 pull-down experiment using anti-His (upper panel) and anti-GST (middle and lower panels) antibodies. Extracts of E. coli cells expressing recombinant His-tagged zebrafish (Danio rerio, Dr) Washc4 (His-DrWashc4) were incubated with either no beads (ctrl) or glutathione beads carrying GST (GST), GST–DrVcp wt or GST–DrVcpR155H. Coomassie stained SDS–PAGE gel of a Washc4 affinity isolation experiment (lower panel). ctrl = beads incubated with PBS. (b to b``) Transfection of HEK293T cells using a VCP-eGFP expression construct (b`) and staining with an anti-WASHC4 antibody (b) show cytoplasmic colocalization of both proteins. (c to c``) HEK293T cell immunostaining of CANX (c`) and WASHC4 (c) display colocalization at the endoplasmic reticulum. Scale bar = 5 µm. (d to d``) FDB muscle fiber of a male CD1 mouse transfected with WASHC4-eGFP (d) and VCP-mCherry (d`) expressing constructs demonstrated colocalization of both proteins. (e to e``) Close-ups of the squared area of the FDB muscle fiber of D``. (e```) Line-scan profiles demonstrated overlapping fluorescence intensities. Cell nuclei were counterstained with DAPI. |