- Title
-
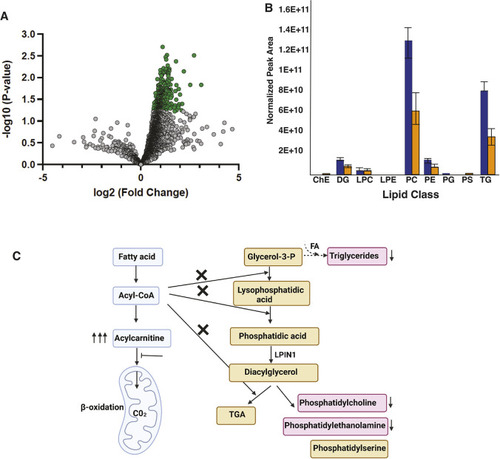
Intrinsic and extrinsic regulation of rhabdomyolysis susceptibility by Tango2
- Authors
- Kim, E.S., Casey, J.G., Tao, B.S., Mansur, A., Mathiyalagan, N., Wallace, E.D., Ehrmann, B.M., Gupta, V.A.
- Source
- Full text @ Dis. Model. Mech.
|
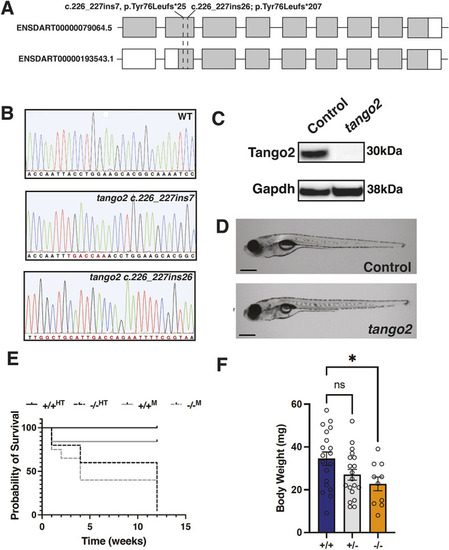
EXPRESSION / LABELING:
|
|
EXPRESSION / LABELING:
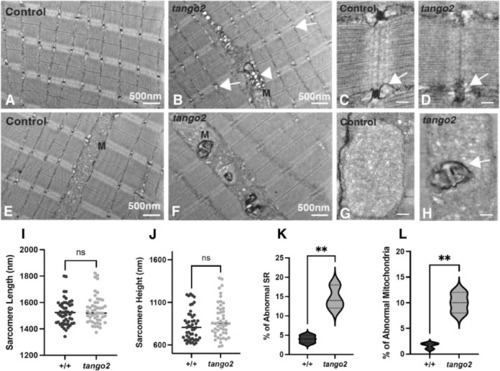
PHENOTYPE:
|
|
PHENOTYPE:
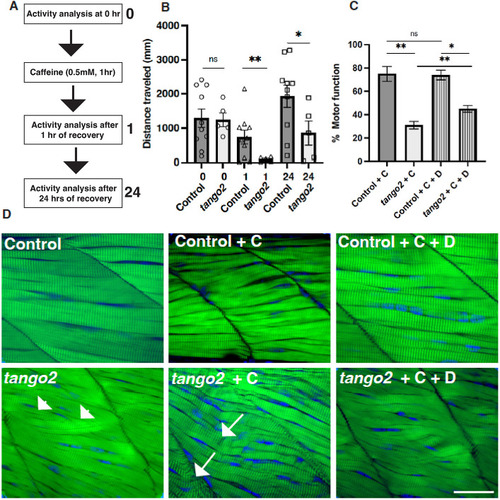
|
|
PHENOTYPE:
|
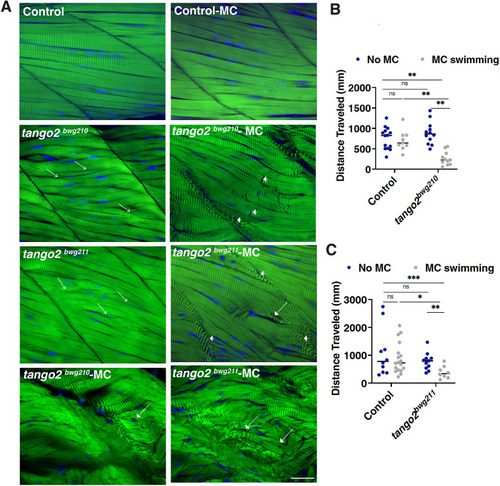
|
PHENOTYPE:
|
|
PHENOTYPE:
|