- Title
-
The Machado-Joseph Disease Deubiquitinase Ataxin-3 Regulates the Stability and Apoptotic Function of p53
- Authors
- Liu, H., Li, X., Ning, G., Zhu, S., Ma, X., Liu, X., Liu, C., Huang, M., Schmitt, I., Wüllner, U., Niu, Y., Guo, C., Wang, Q., Tang, T.S.
- Source
- Full text @ PLoS Biol.
|
ATX-3 promotes p53-dependent apoptosis in cells and in zebrafish. (A) Western blot analysis of the involvement of ATX-3 in apoptosis as measured by the detection of caspase-3 and PARP1 cleavage in ATX-3+/+ and ATX-3-/- MEF cells, transfected with empty vector or plasmid encoding Flag-ATX-3. The relative changing fold of the cleaved PARP1 was underlined. (B) HCT116 p53+/+ and HCT116 p53-/- cells, stably shRNA-expressing cells, or cells transiently transfected with Flag-vector or Flag-ATX-3 or Flag-ATX-3-C14A were treated with or without CPT (1 μM) for 24 h. Cells were analyzed by flow cytometry for apoptosis using Annexin V/PI. Results are the mean ± SEM of three independent experiments. * denotes p < 0.05. Underlying data are shown in S1 Data. (C) WT and p53 mutant zebrafish embryos were injected with indicated object mRNAs (100 pg) at the one-cell stage and harvested at 24 h post fertilization (hpf) for TUNEL labeling and observed by confocal microscopy. Embryos were shown in lateral view with anterior to the left. UIC, uninjected control. (D) Quantitative analysis of Fig 5C. The data represent the mean ± SEM for TUNEL-positive cells in 3~9 zebrafish embryos. ** denotes p < 0.01. Underlying data are shown in S1 Data. |
|
PolyQ expansion affects p53-dependent apoptotic function in cells and in zebrafish. (A) HCT116 p53+/+ and HCT116 p53-/- cells transiently transfected with Flag-vector or normal Flag-ATX-3 or Flag-ATX-3-80Q were treated with CPT (1 μM) for 24 h and then analyzed by flow cytometry for apoptosis using Annexin-V/PI. Results are the mean ± SEM of three independent experiments. * denotes p < 0.05. Cell lysates were immunoblotted with indicated antibodies. Underlying data are shown in S1 Data. (B) HCT116 p53+/+ and HCT116 p53-/- cells transiently transfected with Flag-vector or the normal Flag-ATX-3 or Flag-ATX-3-80Q were treated with CPT (1 μM) for 24 h. Lysates and proteins in the medium were analyzed by immunoblotting with indicated antibodies. M, released into medium; P, pellet. (C and D) Flag-ATX-3-80Q induced p53-dependent apoptosis in zebrafish. Zebrafish embryos were injected with 100 pg of Flag-ATX-3 (ATX-3) or Flag-ATX-3-80Q (ATX-3-80Q) mRNA at one-cell stage. UIC, uninjected control. Twenty-four hpf, embryos were subjected to TUNEL assay and observed by confocal microscopy. Endogenous p53 levels in the p53 WT and p53 mutant zebrafish were examined by western blot. (C) TUNEL-positive cells were quantified from 5~7 embryos each. Results are the mean ± SEM. ** denotes p < 0.01, compared with the control. (D). Underlying data are shown in S1 Data. (E) Whole-mount in situ hybridization analyses of the midbrain neural marker otx2 in uninjected control (UIC) or normal ATX-3 (WT) or ATX-3exp (80Q) mRNA-injected p53 WT (left) and p53 mutant (right) zebrafish embryos at 24, 48, and 72 hpf. Embryos were shown in lateral views with anterior to the left. The ratio of embryos with the representative phenotypes was indicated. Both WT and 80Q mRNA injections resulted in obvious otx2 signal decreases in p53 WT but not p53 mutant zebrafishes, with more profound otx2 signal reduction in 80Q mRNA injections. MB denotes midbrain. EXPRESSION / LABELING:
|
|
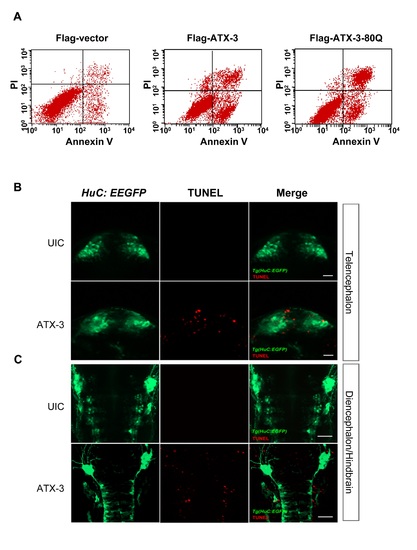
Ataxin-3 expression-induced cell death occurs in cells and in HuC positive brain regions in zebrafish. (A) Flow cytometry analysis using Annexin V-FITC/PI staining in HCT116 p53+/+ cells. (B and C) Dorsal views with anterior to the top of Tg(HuC:EGFP) embryos. Colocalization of HuC:EGFP (green) and TUNEL positive foci (red) in the telencephalon region (B) and diencephalon/hindbrain (C). Tg(HuC:EGFP) transgenic embryos uninjected control (UIC) or injected with ataxin-3 were collected for TUNEL staining at 24 hpf. Scale bars, 20 μm for B and 50 μm for C. EXPRESSION / LABELING:
|
|
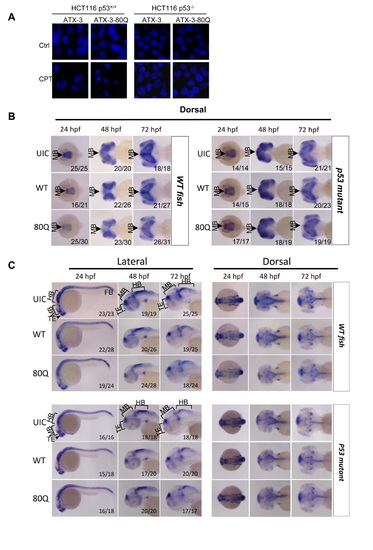
PolyQ expansion in ataxin-3 induces p53-dependent neurodegeneration in zebrafish. (A) Normal, apoptotic and late apoptotic/necrotic cells were observed by staining of nuclear DNA with Hoechst-33342 under fluorescence microscopy. HCT116 p53+/+ and HCT116 p53-/- cells transiently transfected with Flag-ataxin-3-80Q or Flag-ataxin-3 were left untreated (upper) or treated with 1μM of CPT (lower) for 24h. Cells were fixed with 4% paraformaldehyde in PBS and their nuclear DNA was stained with Hoechst-33342 for detection of necrosis and apoptosis by morphological features. (B) Whole-mount in situ hybridization analyses of the midbrain neural marker otx2 in uninjected control (UIC) or normal ataxin-3 (WT) or ataxin-3exp (80Q) mRNA-injected WT (left) and p53 mutant (right) zebrafish embryos at 24, 48, and 72 hpf. Embryos were shown in dorsal views with anterior to the left. The ratio of embryos with the representative phenotypes was indicated. Both WT and 80Q mRNA injections resulted in obvious otx2 signal decreases (indicated by arrowheads) in wild-type but not p53 mutant zebrafishes, with more profound otx2 signal loss in 80Q mRNA injections. MB denotes midbrain. (C) Whole-mount in situ hybridization analyses of the central nervous system marker ngn1 in UIC or normal ataxin-3 (WT) or ataxin-3exp (80Q) mRNA injected-WT (upper) and p53 mutant (lower) zebrafish embryos at 24, 48, and 72 hpf. Embryos were shown in both lateral views (left) and dorsal views (right) with anterior to the left. The ratio of embryos with the representative phenotypes was indicated. TE denotes telencephalon; MB denotes midbrain; HB denotes hindbrain. EXPRESSION / LABELING:
|