- Title
-
?-catenin-dependent transcription is central to Bmp-mediated formation of venous vessels
- Authors
- Kashiwada, T., Fukuhara, S., Terai, K., Tanaka, T., Wakayama, Y., Ando, K., Nakajima, H., Fukui, H., Yuge, S., Saito, Y., Gemma, A., Mochizuki, N.
- Source
- Full text @ Development
|
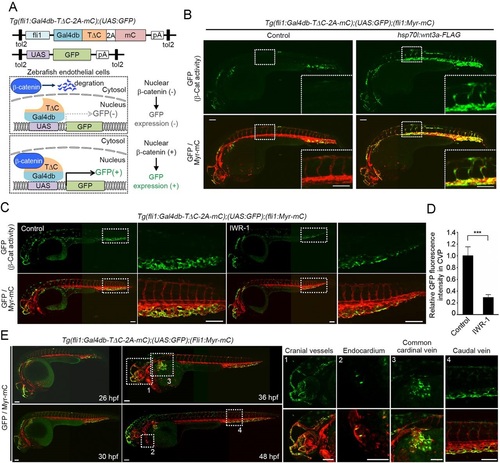
Generation of an endothelial cell-specific β-catenin reporter zebrafish line. (A) Schematic representation of the endothelial cell (EC)-specific β-catenin reporter system. (Top) The constructs used to generate the EC-specific β-catenin reporter zebrafish line; (bottom) how the system works. In the absence of upstream signaling, β-catenin undergoes proteasomal degradation in the cytoplasm. Upon induction of the signaling that promotes β-catenin stabilization, β-catenin translocates to the nucleus and binds Gal4db-TΔC, thereby inducing GFP expression. Thus, this fluorescence reflects the transcriptional activity of β-catenin in ECs. Gal4db, DNA-binding domain of Gal4; TΔC, β-catenin-binding domain of Tcf4; 2A, 2A peptide sequence; mC, mCherry; pA, polyadenylation signal; UAS, upstream activation sequence. (B) 3D-rendered confocal stack fluorescence images of 32hpf Tg(fli1:Gal4db-TΔC-2A-mC);(UAS:GFP);(fli1:Myr-mC) embryos injected without (control) or with hsp70l:wnt3a-FLAG plasmid and heat shocked at 22hpf for 1h. (Top) GFP images (β-catenin activity); (bottom) merged images (GFP/Myr-mC) of GFP (green) and mCherry (red). The boxed areas are enlarged in the insets. All confocal fluorescence images are lateral views with anterior to the left unless otherwise described. Myr-mC, myristoylation signal-tagged mCherry. (C) Confocal images of embryos treated with vehicle (control) or IWR-1, an axin-stabilizing compound, from 15-36hpf, as in B. The boxed areas are enlarged to the right. (D) Fluorescence intensity of GFP in the caudal vein plexus (CVP), as observed in C, relative to that observed in vehicle-treated embryos. Data are meanħs.e.m. Control, n=7; IWR-1, n=11. ***P<0.001. (E) Merged fluorescence images (GFP/Myr-mC) of GFP (green) and mCherry (red) in Tg(fli1:Gal4db-TΔC-2A-mC);(UAS:GFP);(fli1:Myr-mC) embryos at 26, 30, 36 and 48hpf. The boxed areas labeled 1-4 are enlarged to the right, showing (top) GFP images (β-catenin activity) and (bottom) merge of GFP (green) and mCherry (red) (GFP/Myr-mC). Note that green signal that does not overlap with mCherry fluorescence is background autofluorescence of the zebrafish embryos. Panels B, C and E are composites of two or three images, since it was not possible to capture the whole animal at sufficiently high resolution in a single field of view. Scale bars: 100µm. |
|
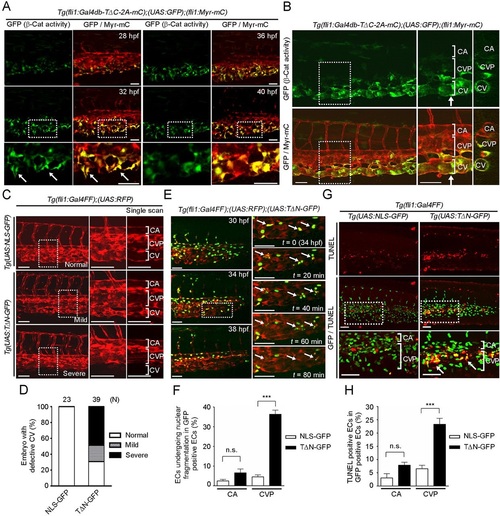
β-catenin-dependent gene expression is required for CV formation. (A) Confocal fluorescence images of a Tg(fli1:Gal4db-TΔC-2A-mC);(UAS:GFP);(fli1:Myr-mC) embryo at 28hpf and subsequent time-lapse images at the indicated time points. (Left) GFP images (β-catenin activity); (right) merge (GFP/Myr-mC) of GFP (green) and mCherry (red). The boxed areas are enlarged beneath. Arrows indicate β-catenin-dependent transcriptionally active ECs that sprout from the CV primordia. (B) Confocal images of the embryo at 48hpf, as in Fig. 1B. The boxed areas are enlarged in the center; transverse sections at the arrows are shown to the right. (C) Confocal stack RFP fluorescence images of 48hpf Tg(UAS:NLS-GFP) and Tg(UAS:TΔN-GFP) embryos with the Tg(fli1:Gal4FF);(UAS:RFP) background. The boxed areas are enlarged (center) and single scanned (right). Note that expression of dominant-negative Tcf, TΔN-GFP, in ECs caused a variety of impairments in CV formation. Embryos with the mild phenotype exhibited no blood circulation in the CV (middle row), whereas those with the severe phenotype lacked the CV (bottom row). Gal4FF, Gal4 DNA-binding domain fused to a duplicated portion of the VP16 transcriptional activation domain; NLS-GFP, nuclear localization signal-tagged GFP. (D) Quantification of the CV phenotypes observed in C, showing the percentage of normal embryos and those with mild and severe phenotypes. The number of embryos analyzed is indicated at the top. (E) Time-lapse confocal imaging of a Tg(fli1:Gal4FF);(UAS:RFP);(UAS:TΔN-GFP) embryo from 30-38hpf. The merged GFP (green) and RFP (red) images at 30, 34 and 38hpf are shown in the left column. In the right column, the boxed area in the 34hpf image is enlarged and subsequent time-lapse images are shown. Arrows indicate ECs undergoing nuclear fragmentation. (F) Percentage of NLS-GFP-expressing and TΔN-GFP-expressing ECs that undergo nuclear fragmentation in the CA and CVP between 30 and 38hpf. Data are expressed as a percentage of the total number of NLS-GFP-expressing and TΔN-GFP-expressing ECs, and shown as meanħs.e.m. NLS-GFP, n=5; TΔN-GFP, n=6. (G) Confocal stack fluorescence images of the 32hpf Tg(fli1:Gal4FF);(UAS:NLS-GFP) (left) and Tg(fli1:Gal4FF);(UAS:TΔN-GFP) (right) embryos with TUNEL staining. Arrows indicate TUNEL-positive ECs that express TΔN-GFP. (H) Percentage of TUNEL-positive cells among the NLS-GFP- or TΔN-GFP-expressing ECs in the CA and CVP at 32hpf. Data are meanħs.e.m. NLS-GFP, n=10; TΔN-GFP, n=9. CA, caudal artery; CV, caudal vein; CVP, caudal vein plexus. (F,H) ***P<0.01; n.s., not significant. Scale bars: 50µm. |
|
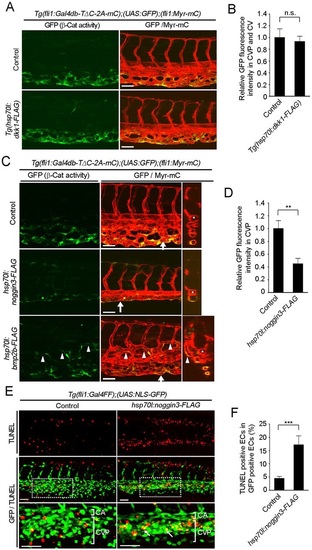
Bmp stimulates β-catenin transcriptional activity in ECs to regulate CV formation. (A) Confocal fluorescence images of 48hpf control (no additional Tg) and Tg(hsp70l:dkk1-FLAG) embryos with the Tg(fli1:Gal4db-TΔC-2A-mC);(UAS:GFP);(fli1:Myr-mC) background heat shocked at 24hpf for 1h. (B) Fluorescence intensity of GFP in the CV and CVP, as observed in A, relative to that in control embryos. Data are meanħs.e.m. Control, n=7; Tg(hsp70l:dkk1-FLAG), n=9. (C) Confocal stack fluorescence images of 36hpf embryos injected without (control) or with hsp70l:noggin3-FLAG or hsp70l:bmp2b-FLAG plasmid and heat shocked at 24hpf for 1h, as in A. Transverse sections at the arrows are shown to the right. Arrowheads indicate ectopic venous vessels originating from the CVP. Asterisks indicate CA. (D) Fluorescence intensities of GFP in the CVP of control and hsp70l:noggin3-FLAG-injected embryos, as observed in C, relative to that in control embryos. Data are meanħs.e.m. Control, n=6; hsp70l:noggin3-FLAG, n=8. (E) Confocal images of 32hpf Tg(fli1:Gal4FF);(UAS:NLS-GFP) embryos injected without (control) or with hsp70l:noggin3-FLAG plasmid, heat shocked at 24hpf and subjected to TUNEL staining, as in Fig. 2G. (F) Percentage of TUNEL-positive cells among NLS-GFP-expressing ECs in the CVP of the control and hsp70l:noggin3-FLAG-injected embryos as observed in E. Data are meanħs.e.m. Control, n=15; hsp70l:noggin3-FLAG, n=15. (B,D,F) **P<0.01, ***P<0.001; n.s., not significant. Scale bars: 50µm. |
|
Aggf1 is involved in Bmp-mediated CV formation. (A) Expression patterns of aggf1 mRNA in zebrafish embryos at 24, 30 and 36hpf, as detected by whole-mount in situ hybridization. Sense probe was used to confirm the specificity of the hybridization reaction. Regions from the yolk tube to the tail are enlarged beneath. (B) Expression of aggf1 mRNA in 36hpf embryos injected without (control) or with hsp70l:noggin3-FLAG or hsp70l:bmp2b-FLAG plasmid and heat shocked at 24hpf. The caudal regions are enlarged to the right. (C) Confocal stack GFP images of the caudal region of 48hpf Tg(fli1:GFP) embryos injected with control morpholino oligonucleotide (MO) or two independent MOs against aggf1 (MO1 and MO2). Single-scan confocal images of these embryos are shown to the right. (D) The CV phenotypes observed in C were quantified as in Fig. 2D. (E) Confocal stack images of the caudal region of 48hpf Tg(fli1:GFP) embryos injected with hsp70l:bmp2b-FLAG plasmid together with either control MO or aggf1 MO1. Arrowheads indicate ectopic venous vessels originating from the CVP. (F) Quantification of ectopic venous vessel formation observed in E, showing the area covered by ectopic venous vessels relative to that in control MO-injected embryos. Data are meanħs.e.m. Control MO, n=10; aggf1, MO1 n=10. (G) Confocal stack fluorescence images of 32hpf Tg(fli1:Gal4FF);(UAS:NLS-GFP) embryos injected with control MO or aggf1 MO1 and subjected to TUNEL staining, as in Fig. 2G. (H) Percentage of TUNEL-positive ECs among the NLS-GFP-expressing ECs in the CVP of embryos injected with either control MO or aggf1 MO1 as observed in G. Data are meanħs.e.m. Control MO, n=16; aggf1 MO1, n=16. (F,H) *P<0.05, ***P<0.001; n.s., not significant. Scale bars: 50µm. |
|
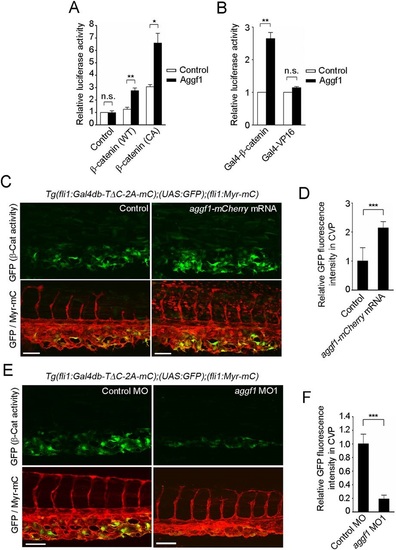
Aggf1 functions downstream from Bmp to stimulate β-catenin transcriptional activity during CV formation. (A) Relative TOPflash/FOPflash activity in HEK 293 cells transfected with empty vector (control) or plasmid encoding either wild-type (WT) or constitutively active (CA) β-catenin together with the empty plasmid (control) or that expressing Aggf1. Data are shown relative to that in the empty vector-transfected cells, as the meanħs.e.m. of three independent experiments. (B) Relative luciferase activity in HEK 293 cells transfected with UAS-luciferase reporter and plasmid encoding either Gal4-β-catenin or Gal4-VP16 together with the empty plasmid (control) or that expressing Aggf1. Data are relative to that observed in the empty vector-transfected cells that express Gal4-β-catenin or Gal4-VP16, as the meanħs.e.m. of three independent experiments. (C) Confocal images of caudal regions of 36hpf Tg(fli1:Gal4db-TΔC-2A-mC);(UAS:GFP);(fli1:Myr-mC) embryos injected without (control) or with aggf1-mCherry mRNA, as in Fig. 1B. (D) Fluorescence intensity of GFP in the CVP, as observed in C, relative to that observed in control embryos. Data are meanħs.e.m. Control, n=9; aggf1-mCherry mRNA, n=8. (E) Confocal images of caudal regions of 36hpf Tg(fli1:Gal4db-TΔC-2A-mC);(UAS:GFP);(fli1:Myr-mC) embryos injected with control MO or aggf1 MO1, as in Fig. 1B. (F) Fluorescence intensity of GFP in the CVP, as observed in E, relative to that observed in control MO-injected embryos. Data are meanħs.e.m. Control MO, n=11; aggf1 MO1, n=12. (A,B,D,F) *P<0.05, **P<0.01, ***P<0.001; n.s., not significant. Scale bars: 50µm. EXPRESSION / LABELING:
|
|
Bmp regulates CV formation by inducing Nr2f2 expression via β-catenin. (A-E) Expression patterns of nr2f2 mRNA in the caudal regions of 48hpf embryos. (A) Embryos injected without (control) or with hsp70l:noggin3-FLAG plasmid were heat shocked at 24hpf. (B) Embryos injected without (control) or with hsp70l:bmp2b-FLAG plasmid were heat shocked at 24hpf. (C) Tg(fli1:Gal4FF);(UAS:NLS-GFP) and Tg(fli1:Gal4FF);(UAS:TΔN-GFP) embryos. (D) Embryos injected with control MO or aggf1 MO. (E) Control embryos (top left) were injected with aggf1-mCherry mRNA, or incubated with BIO (a glycogen synthase kinase 3 inhibitor), or injected with aggf1-mCherry mRNA and then treated with BIO. (F-H) Confocal stack images of the caudal regions of Tg(fli1:GFP) embryos injected with control MO or nr2f2 E1I1 MO at 28 (F), 34 (G) and 48 (H) hpf. The boxed areas are enlarged to the right. Note that knockdown of Nr2f2 caused a variety of impairments in CV formation. Embryos with the mild phenotype lacked the CV, but developed the CVP (middle row in H), whereas those with severe phenotypes lacked the CV and exhibited defective CVP (bottom row in H). (I) CV phenotypes observed in F were quantified, as in Fig. 2D. (J,K) Expression patterns of flt4 (J) and fli1a (K) mRNAs in 36hpf embryos injected with control MO or nr2f2 E1I1 MO. Regions from the yolk tube to the tail are enlarged beneath. Scale bars: 100µm. |